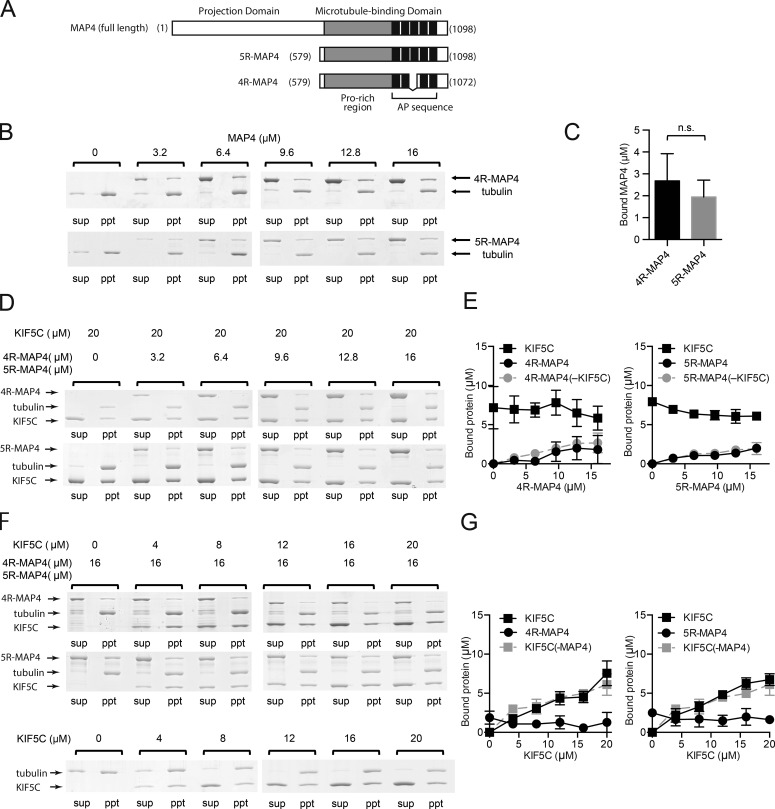
Figure 1.
MAP4 and kinesin-1 can be simultaneously bound to the microtubules. (A) Primary structure of MAP4 and its microtubule-binding fragments 5R-MAP4 and 4R-MAP4. (B–G) Microtubule cosedimentation assays. All experiments were performed three independent times, and the concentrations of bound protein were calculated from each SDS-PAGE gel. Error bars in graphs represent SD (n = 3). (B) Typical SDS-PAGE gels of microtubule-binding assays of 4R- or 5R-MAP4 without kinesin-1. (C) Concentrations of bound MAP4 fragments when 16 µM 4R- or 5R-MAP4 was incubated with microtubules (in the absence of kinesin-1). There was no significant difference between the bound 4R- and 5R-MAP4. (D) Typical results for binding of 4R- or 5R-MAP4 to microtubules in the presence of 20 µM kinesin-1. (E) The concentrations of bound 4R- or 5R-MAP4 and kinesin-1 in the presence of 20 µM kinesin-1. The concentrations of bound 4R- or 5R-MAP4 in the absence of kinesin-1 are also shown. (F) Typical results of microtubule-binding assays of kinesin-1 with 16 µM 4R- or 5R-MAP4. Sup, supernatant; ppt, precipitate. (G) The concentrations of bound kinesin-1 and 4R- or 5R-MAP4. The concentrations of bound kinesin-1 in the absence of MAP4 are also shown.