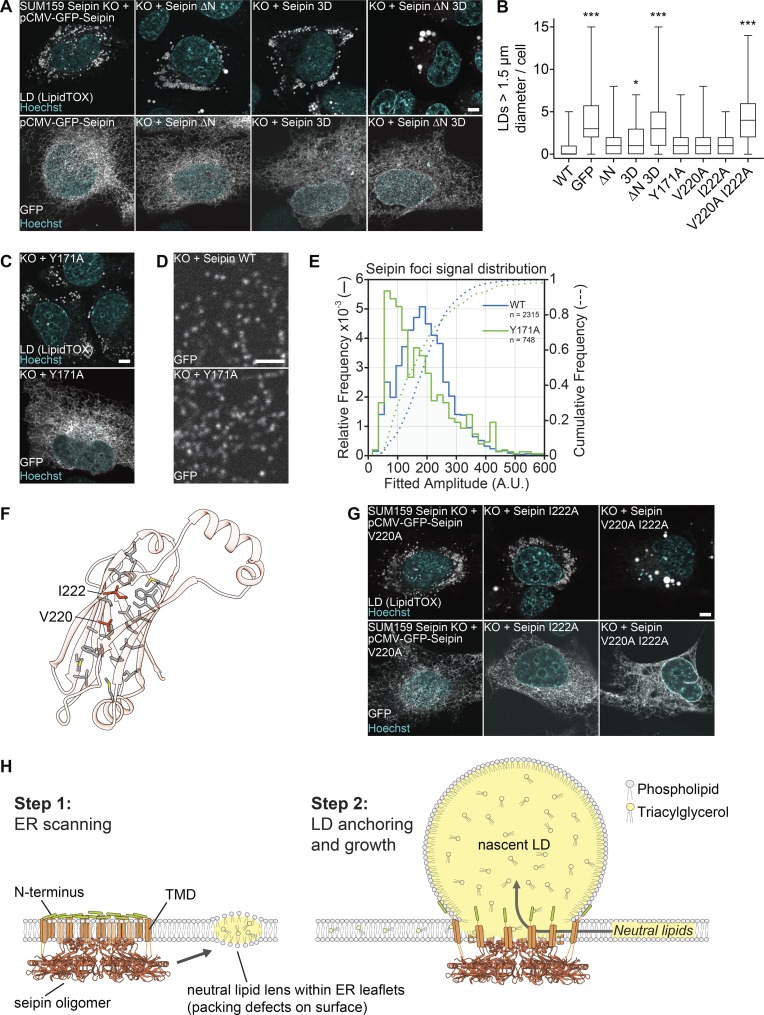
Figure 4.
Testing key features of the seipin luminal domain in cells. (A) The hydrophobic and N-terminal helices are required for seipin function. SUM159 seipin-knockout (KO) cells were transfected with seipin constructs with N-terminal GFP and analyzed for LD phenotype after 24 h oleate treatment. Top: LipidTOX staining. Bottom: Localization to the ER using GFP fluorescence. Representative images are shown. (B) LD size of ≥47 transfected cells per construct from experiments in A, C, and G represented as boxplots. *, P < 0.01; ***, P < 0.0001 compared with WT sample. (C) Seipin Y171A mutant rescues seipin deficiency. Transfection and cell treatment as in A. Top: LD phenotype. Bottom: Localization to the ER. (D) Seipin WT and Y171A form fluorescent foci in the ER. Cells were imaged without oleate addition. To monitor puncta, low transfected cells were monitored. (E) Seipin puncta of 38 cells expressing seipin WT and 31 cells expressing seipin Y171A as in D were tracked and quantified over time. The comparative foci signal distributions are shown for seipin WT (blue lines) and Y171A (green lines). (F) Hydrophobic residues form a putative pocket in the luminal domain of seipin. Residues mutated in B and G are indicated. (G) Analysis of dmSeipin luminal domain mutants as in A. Bars: 5 µm (A, C, and G); 2 µm (D). (H) Model for the molecular function of seipin during LD formation. TMD, TM domain.