Abstract
Objective:
Adolescent girls and young women (AGYW) have a much higher risk of HIV infection than young men of the same age. One hypothesis for this disparity is AGYW are more likely to be in sexual partnerships with older men with HIV; however, evidence has been inconclusive.
Design:
We used longitudinal data from a randomized trial in South Africa (HPTN 068) to determined whether partner age difference is associated with incident HIV infection in AGYW.
Methods:
Age difference was examined continuously and dichotomously (≥5 years). We examined inverse probability of exposure weighted survival curves and calculated time-specific risk differences and risk ratios over 5.5 years of follow-up. We also used a marginal structural Cox model to estimate hazard ratios over the entire study period.
Results:
Risk of HIV was higher in AGYW with an age-disparate partnership versus not and the risk difference was largest at later time points. At 5.5 years, AGYW with an age-disparate partnership had a 12.6% (95% confidence interval 1.9–23.3) higher risk than AGYW with no age-disparate partnerships. The weighted hazard ratio was 1.91 (95% confidence interval 1.33–2.74), an association that remained after weighting for either transactional or condomless sex, and after examining continuous age-differences.
Conclusion:
Age-disparate partnerships increased risk of HIV infection, even after accounting for transactional sex and condomless sex. The relationship between age-disparate partnerships and HIV infection may be explained by increased exposure to infection from men in a higher HIV prevalence pool rather than differences in sexual behaviour within these partnerships.
Keywords: adolescent and young women, age-disparate partnerships, HIV, South Africa
Introduction
In rural South Africa, the prevalence of HIV among adolescent girls and young women (AGYW) aged 15–24 years is 16% and is around five times as high as the prevalence in young men of the same age [1]. One hypothesis to explain this disparity is that young women frequently are in sexual partnerships with older men, who are more likely to be infected with HIV [2–4] and who are more likely to transmit HIV to AGYW due to risky sexual behaviours, including condomless sex [3,5–7], transactional sex [2] and having concurrent sexual partnerships with other women [4].
Evidence from cross-sectional and ecological studies suggest that age-disparate partnerships (in which the male partner is 5 or more years older) contribute to the high risk of HIV among AGYW in sub-Saharan Africa [8–13]; however, findings from longitudinal studies have been mixed. Two studies – a population-based study of women ages 15–29 years in rural KwaZulu-Natal, South Africa[14], and a study of South African women ages 18–45 years enrolled in the VOICE microbicide trial [15] – both found that age-disparate partnerships were not associated with increased risk of incident HIV infection. Although more recent longitudinal evidence, including two studies in South Africa, support positive associations between older partner age and incident HIV infection. The first, a phylogenetic study, demonstrated that AGYW ages 15–24 years are being infected by older male partners [16]. The second, a population-based study, found that HIV incidence was highest among 15 to 24-year-old women with partners ages 30–34 years [incidence rate=9.7, 95% confidence interval (95% CI), 7.2–13.1], followed by partners ages 25–29 years (incidence rate=8.2, 95% CI,7.2–9.4) [13]. These findings are also supported by study of young women ages 15–24 years in Zimbabwe, which found that increasing continuous partner age difference (adjusted hazard ratio =1.05, 95% CI: 1.01–1.09) and intergenerational partnerships (≥10 years difference) were associated with increased HIV incidence [adjusted hazard ratio (aHR) = 1.78, 95% CI: 0.96–3.29] [17]. The study did not find an association with intragenerational partnerships (5 to 9-year difference). Differences between the studies may be due to not controlling for important variables, including transactional sex; differences in population characteristics, including age, school enrolment, and HIV prevalence; and differences in study methodologies, including less private data collection approaches.
The aim of the current study is to determine whether age-disparate partnerships are associated with an increased risk of incident HIV infection among sexually active AGYW in rural South Africa and to examine how transactional sex and condomless sex affect this relationship.
Materials and methods
Study population
We use data collected during the HIV Prevention Trials Network (HPTN) 068 study, a randomized trial designed to examine whether providing cash transfers, conditional on school attendance, reduced risk of HIV acquisition in AGYW [18,19]. We used these data to estimate the association between having an age-disparate relationship and incident HIV infection. The study enrolled 2533 AGYW age 13–20 years who were enrolled in high school grades 8–11 in the rural Bushbuckridge subdistrict of Mpumalanga Province, South Africa in 2011 [18]. The study area has a high HIV prevalence and high levels of poverty, unemployment and migration for work [20]. AGYW who were pregnant or married or had no parent/guardian in the household were not eligible for study enrolment. To assess incident HIV infection, we included AGYW from the original study who were HIV negative at enrolment and had at least one other follow-up test. In addition, we only included AGYW who reported ever having had sex before or during the study. As a sensitivity analysis, we included all AGYW including those who reported never having had sex. The sensitivity analysis was done to ensure similar results given the high number of incident HIV cases in AGYW who reported never having had sex (44 infections).
During the main trial (2011–2014), AGYW were seen annually from enrolment until study completion or graduation from high school, whichever came first. An additional survey was done after the main trial around 1 or 2 more years postintervention (‘postintervention visit’), adding to a total of up to 6 years of possible exposure time. During the main study period, participants could have up to three follow-up visits at roughly every 12 months. An additional HIV test was also done for some AGYW around the time of the AGYW’s expected graduation from high school or when the study was completed to capture more person time (termed the ‘graduation test’). This test was typically around 6 months after the previous annual visit and was before the postintervention visit, which occurred roughly 1–2 years after the intervention ended. Each study visit, with the exception of the graduation test visit, included an audio computer-assisted self-interview (ACASI) with the AGYWand her parent/guardian and HIVand herpes simplex virus type-2 (HSV-2) testing for those who were negative at the previous visit. The graduation test visit included only an HIV and HSV-2 test.
Exposure, outcome and covariate ascertainment
Partner age difference was calculated as the difference between the AGYW’s age and the self-reported age of her oldest sexual partner (up to three partners) at each follow-up visit. The binary, time-varying exposure of having an age-disparate partnership was defined as having at least one sexual partner five or more years older at each study visit. Girls who did not report a partner at a given visit were coded as missing partner age in the main analysis. In the sensitivity analysis with all girls, girls without a partner were coded as not having an age-disparate partner. In addition, we examined partner age-difference continuously by taking the mean and maximum age difference from all reported partners. Because the results were similar, we report only maximum age difference. As a sensitivity analysis, we included all AGYW regardless of sexual activity and calculated age-difference exposures using the self-reported age of both sexual (up to three) and nonsexual (one, if not sexually active) partners. In addition, we ran another sensitivity analysis using sexual behaviours from the time period prior to HIV infection to further account for potential reverse causality due to not knowing the exact timing of HIV infection. The outcome of incident HIV infection was defined as new cases of HIV following study enrolment. Testing for HIV infection was done using two different HIV rapid tests: the Determine HIV-1/2 test (Alere Medical Co., Ltd, Matsudo-shi, Chiba, Japan) and the FDA-cleared Uni-gold Recombigen HIV test (Trinity Biotech plc, Bray, Co. Wicklow, Ireland)). Confirmatory HIV testing was performed using the FDA-cleared GS HIV-1 WESTERN BLOT assay (Bio-Rad Laboratories, Inc. Redmond, Washington, USA) [19]. HSV-2 testing was done using the HSV-2 IgG ELISA assay (Kalon Biological, Ltd, Guildford, UK) [21]. Testing was done at the site and confirmed at the HPTN Laboratory Center.
We used a directed acyclic graph (DAG) to select a minimally sufficient adjustment set of both time-fixed and time-varying covariates. Potential confounders were included in our DAG based on prior literature (Appendix Figure 1, http://links.lww.com/QAD/B371). Confounders in the adjustment set were age at baseline, time-varying self-report of any alcohol use and time-varying enrolment or completion of secondary school. If a young woman was enrolled in school at a visit or had completed grade 12, she was defined as being enrolled or having completed school. We also adjusted for intervention assignment at baseline to account for study design; however, the cash transfer intervention did not have an effect on incident HIV infection. We examined models adjusted for wealth, depression and low sexual relationship power score (SRPS) but did not include these in the final model, as results were similar. Depression was defined as having a children’s depression inventory score at least 7 at baseline [22] or a Center for Epidemiologic Studies Depression (CESD) score at least 16 at any follow-up visit [23]. Relationship power was assessed using the South African adaptation of the Sexual Relationship Power Scale (SRPS) [24]. High power was defined as having an SRPS score in the top two-thirds of the distribution.
As an exploratory analysis, we also examined models that accounted for any condomless sex in the last 3 months and transactional sex (given money or gifts in exchange for sex), both self-reported. Descriptively, we looked at wealth in quartiles according to household assets, self-report of risky sexual behaviours (any condomless sex, transactional sex and number of partners in the last 12 months) and revised children’s manifest anxiety score[25], and depression, and high sexual relationship power score [24,26].
Because ACASI information was not collected at the graduation visit, we kept only HIV test information from graduation visits that were within 6 months of the previous visit and carried forward ACASI data from the last visit. We did this to minimize bias in carrying the last observation forward [27], assuming that information within 6 months would be similar to what was reported at the previous visit. For all other study visits, we carried forward data missing covariate information from the last observation, as we did not expect answers to vary dramatically and missing data were minimal (<10%). We did not carry forward exposure or sexual behaviour information, except as described above. We only used observations with complete cases for the exposure, but missing data were minimal (<10%).
Statistical analysis
To estimate the effect of having an age-disparate partnership on time to incident HIV infection, we used inverse probability-of-exposure weighted survival curves and a marginal structural Cox model [28]. The origin for each AGYW was the date where she became sexually active. As sexual initiation would have occurred sometime between surveys, the time she entered the risk set was the time of the last survey. If she was sexually active at baseline, this date was the date of enrolment in the study. Time was modelled in continuous months from this date of origin until date of detection of HIV infection, date of visit before loss to follow up, outmigration or administrative censoring at the last visit.
We estimated crude and weighted cumulative incidence (risk) of incident HIV infection using the extended Nelson–Aalen estimator with inverse probability of exposure weights (IPW) [29,30]. IPW was used to produce curves for the cumulative incidence of HIV infection comparing having an age-disparate partnership ( 5 years) to not having an age-disparate partnership, standardized to the covariate distribution in the entire study sample to account for confounding [31,32].
We then used these curves to calculate standardized risk ratios and risk differences comparing cumulative incidence of HIV by age-disparate partnership at different time points (at 1.5, 2.5, 3.5, 4.5 and 5.5 years). We used5.5 instead of 6 years (total) follow-up time as the risk set became small after 5.5 years. CIs were calculated for the survival curves using the standard deviation of a nonparametric bootstrap calculated from 200 full samples (with replacement) from the observed data [33].
Cox proportional hazards models were used to compare hazards of incident HIV infection by age-disparate partnership over the entire study period. The Efron method [34] was used to account for tied data and the Cox proportional hazards assumption was assessed by including a product term between the exposure and time; we found no violation [35]. In addition, we examined effect modification by age at study entry [i.e. first sexual activity; (age 13–14, 15–16, 17–18 and 19+ years], school status (in school versus out of school) and visit by fitting a different model for each subgroup.
We accounted for confounding in the cox model and survival curves using IPW [36], which were calculated using a pooled logistic regression model for the exposure conditional on confounders [37,38]. To increase the efficiency of our estimator, weights were stabilized and were examined descriptively to ensure a mean of one [36]. For continuous maximum age difference, we binned the exposure into five groups before creating weights [39]. To adjust for potentially informative censoring due to differential loss to follow up by AGYW age and exposure status, we multiplied our IPW by time-varying inverse probability-of-censoring weights. Pooled logistic regression models analogous to the previous IPW were used to estimate censoring weights [28,38,40]. Censoring weights included the exposure and age at baseline to account for potential differential loss to follow-up. SAS version 9.3 (SAS Institute, Inc., Cary, North Carolina, USA) was used for all analyses.
This study was primarily funded by awards from the National Institutes of Health. The funder had no role in the analysis of data, interpretation of results, writing of report or decision to submit for publication. The original study design was reviewed, approved and overseen by the funder [19]. The corresponding author had full access to the data and made the final decision to submit for publication.
Results
From the 2533 young women enrolled in the original trial, our analysis included 1626 AGYW who were HIV negative at baseline, had at least one follow-up HIV test and reported ever having had sex before or during the study period. A total of 146 incident HIV cases occurred during the study. At baseline or the first visit where AGYW became sexually active, the median age was 16 years, 94.0% were enrolled or had completed high school, 5.7% were a double or single orphan, and 32.5% had ever been pregnant (Table 1). Roughly 19% (N = 304) reported having an age-disparate partnership (≥5 years); these AGYW were more likely to have ever been pregnant, have condomless sex, have more than one partner in the last 12 months, be infected with HSV-2, have transactional sex and report low sexual relationship power. We did not find differences by age, wealth, randomization arm, school enrolment or completion, orphan status, alcohol use or depression. After IPW weighting, covariates were balanced by exposure status.
Table 1.
Characteristics of HIV-negative young women aged 13–26 years at baseline or the visit where they first become sexually active by age-disparate partnership in Agincourt, South Africa from 2011 to 2017 (N = 1626).a
| Age-disparate partnership | ||||||
|---|---|---|---|---|---|---|
| No (N = 1292, 81.0%) | Yes (N = 304, 19.0%) | Total (N = 1626) | ||||
| N (%) | Median (IQR) | N (%) | Median (IQR) | N (%) | Median (IQR) | |
| Young women’s age at baseline (years) | 16 (15–17) | 16 (14–17) | 16 (15–17) | |||
| Age 13–14 | 295 (22.8) | 85 (28.0) | 380 (23.8) | |||
| Age 15–16 | 575 (44.5) | 139 (45.7) | 714 (44.7) | |||
| Age 17–18 | 335 (25.9) | 67 (22.0) | 402 (25.2) | |||
| Age 18–20 | 87 (6.7) | 13 (4.3) | 100 (6.3) | |||
| Household socioeconomic status | ||||||
| Low | 257 (20.0) | 57 (18.9) | 314 (19.8) | |||
| Middle to Low | 326 (25.4) | 60 (19.9) | 386 (24.4) | |||
| Middle | 357 (27.8) | 105 (34.9) | 462 (29.1) | |||
| High | 344 (26.8) | 79 (26.3) | 423 (26.7) | |||
| Intervention arm | 649 (50.2) | 154 (50.7) | 803 (50.3) | |||
| Enrolled or completed school | 1120 (94.4) | 280 (92.1) | 1500 (94.0) | |||
| Maximum partner age difference (years) | 2 (1–3) | 6 (5–7) | 2 (1 −4) | |||
| Double orphan | 74 (5.8) | 13 (4.3) | 87 (5.7) | |||
| Ever pregnant | 372 (29.9) | 125 (43.6) | 497 (32.5) | |||
| Any condom less sex acts in the last 3 months | 280 (22.0) | 117 (39.3) | 397 (25.3) | |||
| Number of sex partners in the last 12 months | ||||||
| Zero | 90 (7.1) | 10 (3.3) | 100 (6.4) | |||
| One | 1008 (80.0) | 192 (64.2) | 1200 (77.0) | |||
| More than one | 162 (12.9) | 97 (32.4) | 259 (16.6) | |||
| Received money or gifts in exchange for sex | 226 (20.3) | 78 (27.7) | 304 (21.8) | |||
| Prevalent HSV-2 infection | 80 (6.3) | 28 (9.5) | 108 (6.9) | |||
| Any alcohol use | 160 (12.5) | 49 (16.3) | 209 (13.3) | |||
| Depressionb | 378 (30.2) | 97 (33.0) | 475 (30.7) | |||
| Children’s manifest anxiety score >7 | 247 (26.4) | 72 (28.8) | 319 (26.9) | |||
| High Sexual Relationship Power Score (SRPS) | 622 (49.5) | 123 (42.3) | 491 (43.0) | |||
Missing; partner number 37; socioeconomic status 11; ever repeat 0; orphan 12; unprotected sex 27; pregnant 66; alcohol 19; HSV-2 22; anxiety 410; depression 50; exchange sex 199; low power 49; partner age difference 30.
Children’s depression index score ≥ 7 or Center for Epidemiologic (CESD) Studies of Depression Score ≥ 16.
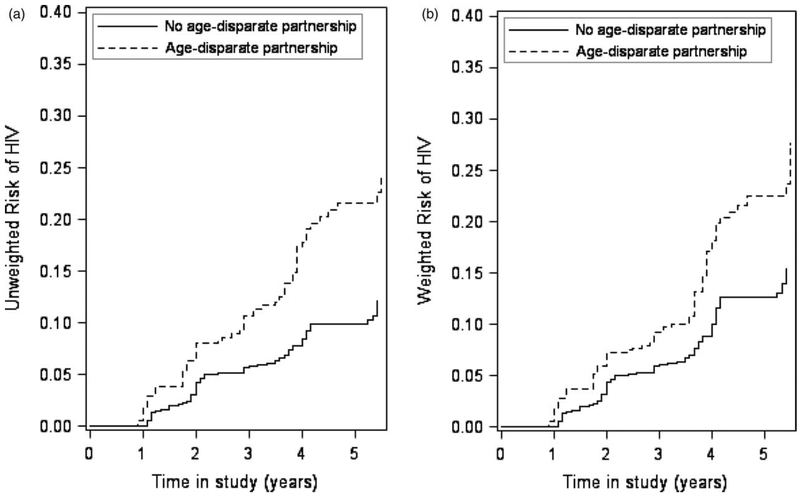
Risk of incident HIV infection increased over the study period and was higher for girls with an age-disparate partnership compared with not (Fig. 1). The difference between the curves was largest at the end of the follow-up period with differences becoming more pronounced at 4 years. This increased difference corresponds to timing of the postintervention visit when AGYW were older and the majority had left or completed school. At 5.5 years after enrolment, the standardized (accounting for confounding) risk of HIV in AGYW with an age-disparate partnership was 29.5% compared with 16.9% in those without an age-disparate partnership for a standardized risk difference of 12.6% (95% CI 1.9–23.3) and standardized risk ratio of 1.74 (95% CI 1.13–2.68) (Table 2).
Fig. 1. Cumulative incidence curves for the association between age-disparate partnership and incident HIV infection for 1626 sexually active young women with no prevalent HIV infection from HPTN 068 conducted in 2011–2017.
(a) Unweighted; (b) weighted. Exposure weighted curves accounted for the following covariates: age at baseline, time-varying school enrolment or completion, time-varying alcohol use, intervention assignment at baseline.
Table 2.
1.5 to 5.5-year weighted risk differences and risk ratios for the effect of age-disparate partnership on incident HIV infection among sexually active young women without prevalent infectiona.
| No. infected | Risk (%) | Risk difference (%) | (95% CI, %) | Risk Ratio | (95% CI) | |
|---|---|---|---|---|---|---|
| Year 1.5 | 24 | |||||
| Not age-disparate (<5 years) | 17 | 2.0 | 0 | 0 | 1 | 1 |
| Age-disparate (≥5 years) | 7 | 3.7 | 1.7 | (−1.2 to 4.6) | 1.86 | (0.70–4.91) |
| Year 2.5 | 62 | |||||
| Not age-disparate (<5 years) | 43 | 5.1 | 0 | 0 | 1 | 1 |
| Age-disparate (≥5 years) | 19 | 7.6 | 2.5 | (−1.6 to 6.6) | 1.49 | (0.81–2.75) |
| Year 3.5 | 82 | |||||
| Not age-disparate (<5 years) | 53 | 6.7 | 0 | 0 | 1 | 1 |
| Age-disparate (≥5 years) | 29 | 10.2 | 3.5 | (−1.0 to 8.0) | 1.51 | (0.91–2.52) |
| Year 4.5 | 126 | |||||
| Not age-disparate (<5 years) | 74 | 12.7 | 0 | 0 | 1 | 1 |
| Age-disparate (≥5 years) | 52 | 21.6 | 9.0 | (1.8–16.2) | 1.71 | (1.14–2.55) |
| Year 5.5 | 146 | |||||
| Not age-disparate (<5 years) | 84 | 16.9 | 0 | 0 | 1 | 1 |
| Age-disparate (≥5 years) | 62 | 29.5 | 12.6 | (1.9–23.3) | 1.74 | (1.13–2.68) |
CI, confidence interval.
Weighted for age at baseline, intervention assignment at baseline, time-varying school enrolment or completion, time-varying alcohol use.
Over the study period, 62 incident HIV infections occurred in girls with an age-disparate partnership (≥5 years) and 84 infections were in girls without an age-disparate partnership (Table 3). The weighted hazard ratio was 1.91 (95% CI 1.33–2.74) comparing time to HIV among girls in an age-disparate partnership versus not. The association between having an age-disparate partnership and HIV acquisition remained after weighting to account for transactional sex (hazard ratio 1.85; 95% CI 1.26–2.73) and condomless sex (hazard ratio 1.99; 95% CI 1.22–3.26). A 1-year change in the maximum age difference of all reported sexual partners was associated with a hazard ratio of 1.05 (95% CI 1.03–1.08). Again, results for continuous age difference were similar after adjusting for transactional sex and condomless sex.
Table 3.
Hazard ratios for the effect of age-disparate partnership on incident HIV infection among sexually active young women aged 13–26 years without prevalent infection, enrolled in HPTN 068 from 2011 to 2017a.
| Model | Unweighted no. of events | Unweighted person-months of follow-up | Unweighted HR (95% CI) | Weighted HR (95% Cl)b | Weighted including TS (95% Cl)c | Weighted including condomless sex (95% Cl)b |
|---|---|---|---|---|---|---|
| HIV | ||||||
| Total | 146 | 59 651 | ||||
| Age-disparate (≥5 years) | 62 | 18 857 | 2.27 (1.64–3.14) | 1.91 (1.33–2.74) | 1.85 (1.26–2.73) | 1.99 (1.22–3.26) |
| Not age-disparate (<5 years | 84 | 40 794 | 1 | 1 | 1 | 1 |
| Continuous maximum age difference (1-year change)c | – | – | 1.05 (1.03–1.07) | 1.05 (1.03–1.08) | 1.05 (1.02–1.08) | 1.05 (1.03–1.08) |
CI, confidence interval; HR, hazard ratio.
Weighted models conditioned on the following covariates: age at baseline, time-varying school enrolment or completion, time-varying alcohol use, intervention assignment at baseline.
Three HIV cases missing age difference information, one of these is also missing condomless sex.
Sixteen HIV cases missing transactional sex information (N = 130 incident cases).
The hazard ratio for the effect of having an age-disparate partnership on incident HIV did not vary greatly when examining effect modification by age at study entry (Table 4). However, estimates were more precise for ages 15–16 (46.6%) and 17–18 years (33.6%) when more girls first became sexually active. When examining school status, we found a higher hazard ratio for girls out of school (HR 3.03; 95% CI 1.39–6.58), although there was still an association for girls in school (hazard ratio 1.73; 95% CI 1.14–2.64). When examining hazard ratios by visit, we found the largest effect (hazard ratio 2.60; 95% CI 1.29–5.23) corresponding to the postintervention visit when more girls had older partners ((≥5 years; 28.9%).
Table 4.
Hazard ratios for the effect of age-disparate partnership (≥5 years) on incident HIV infection among sexually active young women aged 13–26 years without prevalent infection, stratified by age at which girls first became sexually active and visita.
| Age-disparate N (% of 4046 visits) | Unweighted HR (95% CI) | Weighted HR (95% CI) | |
|---|---|---|---|
| Overall effect | 2.27 (1.64–3.14) | 1.91 (1.33–2.74) | |
| Stratified by age at study entry (years) | |||
| Age 13–14 | 112 (21.6) | 2.63 (0.85–8.17) | 2.34 (0.78–7.04) |
| Age 15–16 | 414 (22.0) | 2.73 (1.71–4.34) | 2.36 (1.47–3.79) |
| Age 17–18 | 278 (20.3) | 2.33 (1.37–3.96) | 2.15 (1.21–3.84) |
| Age 1 9+ | 52 (18.6) | 1.50 (0.49–4.57) | 1.30 (0.36–4.70) |
| Stratified by school status | |||
| In school | 709 (19.9) | 1.98 (1.37, 2.88) | 1.73 (1.14, 2.64) |
| Out of schoolb | 147 (30.3) | 3.59 (1.76, 7.33) | 3.03 (1.39, 6.58) |
| Stratified by visit | |||
| Main study follow-up 1 | 164 (17.1) | 1.89 (0.91–3.94) | 1.91 (0.91–4.01) |
| Main study follow-up 2 | 204 (19.9) | 1.50 (0.82–3.12) | 1.40 (0.71–2.77) |
| Main study follow-up 3 | 176 (20.6) | 2.96 (1.22–7.18) | 2.37 (0.95–5.91) |
| ’Graduation test visit’ | 86 (20.2) | 1.40 (0.33–5.89) | 1.12 (0.27–4.68) |
| Postintervention visit | 226 (28.9) | 3.48 (1.92–6.32) | 2.60 (1.29–5.23) |
CI, confidence interval; HR, hazard ratio.
Weighted models conditioned on the following covariates: age at baseline, time-varying school attendance, time-varying alcohol use, intervention assignment at baseline.
Out of school for any reason including dropout or completion of school.
In addition, after expanding the sample to include those who reported never having sex during the study, results were similar and more precise, although the hazard ratio for the effect of binary age-disparate partnership was larger and the continuous exposure did not indicate an effect (Appendix Table 1, http://links.lww.com/QAD/B371). Results were also similar in a sensitivity analysis using the exposure from the prior rather than concurrent time period of HIV infection (Appendix Table 2, http://links.lww.com/QAD/B371).
Discussion
Overall, young women with an age-disparate partnership (≥5 years) had an increased risk of incident HIV infection over the study period. The relationship between partner age difference and incident infection was robust when defining age difference continuously or categorically and after adjusting for transactional sex and condomless sex. In addition, risk ratios and risk differences increased over time in the study and we found a greater hazard of HIV infection associated with the postintervention visit and being out of school.
Our results are consistent with several, recent longitudinal studies showing that young women with older partners are more likely to be exposed to HIV infection, including two studies in KwaZulu-Natal, South Africa and one in Zimbabwe [13,16,17]. In Zimbabwe, increasing continuous partner age difference was associated with a modest increase in HIV incidence (adjusted hazard ratio =1.05, 95% CI: 1.01–1.09), which is similar to the estimate reported in our study [17]. However, when age differences were examined categorically, only intergenerational partnerships (≥10 years difference) were associated with increased HIV incidence (aHR=1.78, 95% CI: 0.96–3.29). These findings may be due to the fact that partner age differences of greater than 10 years were rare in our study population (2.6%) but made up 21% of partnerships in the Zimbabwe study.
Researchers previously hypothesized that one possible explanation for the lack of an association between older partner age and incident HIV infection among earlier longitudinal studies may be due to the fact that although older men are more likely to be HIV infected than younger men, younger HIV-infected men are more infectious because they are more likely to be recently infected and less likely to be on ART [14,15]. However, two recent studies examining the association between older partner age and HIV infection among AGYW found that this relationship did not vary during pre versus post-ART eras [17,41]. Moreover, data from the most recent South African National HIV survey (2012) suggest that men in age-disparate partnerships with women ages 15–24 years are more likely to be HIV positive and ART-naive than men in similar-age partnerships [42].
Several key differences may explain conflicting results from earlier longitudinal studies. In the Harling study, partner data were collected only from the most recent sexual partner (which may have biased the sample towards longer partnerships) [14], although in the VOICE trial, partner data were limited to only primary partners (which may have biased the sample towards safer, more socially acceptable partners) [15]. Thus, reported partners in these studies may not represent all sexual partners and may exclude the highest risk partners. In contrast, AGYW in our analysis could report up to three sexual partners at each study visits with no constraints on partner type. The Harling study also used face-to-face interviews by fieldworkers from the local community, rather than more anonymous data collection methods such as ACASI, which may have resulted in social desirability bias [43,44].
Descriptively, we found that AGYW with older partners were more likely to practice other risky sexual behaviours (have a greater number of partners, transactional sex and have condomless sex) and be infected with HSV-2. However, we found that the increase in incident HIV infection due to age-disparate partnerships is not entirely attributable to transactional sex or condomless sex. Rather, in this context, the relationship between partner age and HIV risk is likely due to increased exposure to men in a higher HIV prevalence pool.
Given that the relationship between age-disparate partnerships and HIV acquisition was higher during later time periods, it is critical that HIV prevention interventions address this vulnerable period of transition. Once girls have left or completed school, they are more likely to begin starting a family, have older partners and migrate to seek work or for postsecondary education [45]. HIV prevalence in same age partners and other girls is also higher during this time likely leading to an increased risk of infection through sexual networks. Interventions should include efforts to reduce the risk of HIV transmission to AGYW through biomedical interventions – such as increasing access to preexposure prophylaxis (PrEP) among AGYW with older sexual partners, and increasing HIV testing and rapid linkage, retention in HIV care and sustained viral suppression among men with AGYW sexual partners – and structural interventions such as creating supportive and structured environments wherein AGYW can spend time with peers and develop protective life-skills through vocational training, tertiary education and employment opportunities.
In this study, sexual behaviours were measured using ACASI to minimize the potential for social desirability bias [43]. However, misreporting on sexual behaviours is apparent in the number of incident HIV cases (N=44) among AGYW who reported never having had sex. Because AGYW were tested for HIV after they were interviewed and were not commonly tested outside of the study, it is unlikely that this misreporting is related to HIV status, resulting in bias towards the null. Nonetheless, to address this concern, we conducted a sensitivity analysis using the full AGYW sample, and results were similar (Appendix Table 1, http://links.lww.com/QAD/B371).
There are a few other limitations to our study. First, as the exact timing of HIV infection among AGYW was unknown, HIV infection may overlap with the period of exposure assessment (‘most recent’ reported partners). Again, HIV status was unlikely to influence partner age (resulting in reverse causality) because AGYW were unlikely to know their status and a sensitivity analysis showed results were the same when using exposure from the prior visit. Second, our data are from a single-site and are nested in a randomized trial. Therefore, results may vary in other populations or may have been affected by participation in the trial. For example, given modification by school status, we may expect this relationship to be stronger in populations wherein more girls are out of school. It is also possible that HIV incidence was higher during later time periods due to the end of the cash transfer intervention. However, the cash transfer was not associated with HIV infection or age-disparate partnerships [19]. Lastly, our results assume no unmeasured confounding. Specifically, we estimated the total effect of age-disparate partnerships on incident HIV accounting for measured variables. We did not have information about sexually transmitted infections other than HSV-2, which may be related to HIV acquisition.
Having a relationship with an older partner was associated with an increased risk of incident HIV infection in AGYW. The relationship between age-disparate partnerships and incident HIV remained after accounting for transactional sex and condomless sex and was larger during later time periods. The relationship between partner age and incident HIV infection may be explained by increased exposure to infection by men in a higher HIV prevalence pool. Interventions to prevent HIV should target AGYW who are more likely to be exposed to HIV (e.g. with older partners) and men who have AGYWas sexual partners, and should include biomedical interventions, such as PrEP. Future interventions should consider not only individual behaviours but also factors that are context dependent, including how to intervene in partnership networks to reduce the possibility of exposure to infection.
Acknowledgements
We thank the HPTN 068 study team and all trial participants. The MRC/Wits Rural Public Health and Health Transitions Research Unit and Agincourt Health and Socio-Demographic Surveillance System have been supported by the University of the Witwatersrand, the Medical Research Council, South Africa, and the Wellcome Trust, UK (grants 058893/Z/99/A; 069683/Z/02/Z; 085477/Z/08/Z; 085477/B/08/Z). M.C.D.S., N.N., K.K., A.P. contributed to the conception, design of the analysis and writing of the paper. J.K.E. and J.P.H. provided statistical support on the analysis. The remaining authors were involved in data acquisition, data collection, study management and design of the original parent study. All authors have reviewed the paper, provided comments and edits to the manuscript and have read and approved the final manuscript.
This study was funded by the National Institutes of Health (R01 MH110186, R01MH087118) and by award numbers UM1 AI068619 (HPTN Leadership and Operations Center), UM1AI068617 (HPTN Statistical and Data Management Center) and UM1AI068613 (HPTN Laboratory Center) from the National Institute of Allergy and Infectious Diseases, the National Institute of Mental Health and the National Institute on Drug Abuse of the National Institutes of Health. This work was also supported by the Carolina Population Center and its NIH Center grant (P2C HD050924), National Institute of Health award number T32-MH019139 (Principal Investigator, Theodorus Sandfort, PhD) and by a centre grant from the National Institute of Mental Health to the HIV Center for Clinical and Behavioral Studies at NY State Psychiatric Institute and Columbia University (P30-MH43520; Principal Investigator, Robert Remien, PhD).
Footnotes
Conflicts of interest
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We have no conflicts of interest to declare.
References
- 1.Gómez-Olivé FX, Angotti N, Houle B, Klipstein-Grobusch K, Kabudula C, Menken J, et al. Prevalence of HIV among those 15 and older in rural South Africa. AIDS Care 2013; 25:1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luke N. Age and economic asymmetries in the sexual relationships of adolescent girls in sub-Saharan Africa. Stud Fam Plann 2003; 34:67–86. [DOI] [PubMed] [Google Scholar]
- 3.Luke N Confronting the ‘sugar daddy’ stereotype: age and economic asymmetries and risky sexual behavior in urban Kenya. Int Fam Plan Perspect 2005; 31:6–14. [DOI] [PubMed] [Google Scholar]
- 4.Maughan-Brown B, Kenyon C, Lurie MN. Partner age differences and concurrency in South Africa: implications for HIV-infection risk among young women. AIDS Behav 2014; 18:2469–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jewkes RK, Levin JB, Penn-Kekana LA. Gender inequalities, intimate partner violence and HIV preventive practices: findings of a South African cross-sectional study. Soc Sci Med 2003; 56:125–134. [DOI] [PubMed] [Google Scholar]
- 6.Bankole A, Biddlecom A, Guiella G, Singh S, Zulu E. Sexual behavior, knowledge and information sources of very young adolescents in four sub-Saharan African countries. Afr J Reprod Health 2007; 11:28–43. [PMC free article] [PubMed] [Google Scholar]
- 7.Blanc AK, Wolff B. Gender and decision-making over condom use in two districts in Uganda. Afr J Reprod Health 2001; 5: 15–28. [PubMed] [Google Scholar]
- 8.Kelly RJ, Gray RH, Sewankambo NK, Serwadda D, Wabwire-Mangen F, Lutalo T, et al. Age differences in sexual partners and risk of HIV-1 infection in rural Uganda. J Acquir Immune Defic Syndr 2003; 32:446–451. [DOI] [PubMed] [Google Scholar]
- 9.Gregson S, Nyamukapa CA, Garnett GP, Mason PR, Zhuwau T, Caraël M, et al. Sexual mixing patterns and sex-differentials in teenage exposure to HIV infection in rural Zimbabwe. Lancet 2002; 359:1896–1903. [DOI] [PubMed] [Google Scholar]
- 10.Pettifor AE, Rees HV, Kleinschmidt I, Steffenson AE, MacPhail C, Hlongwa-Madikizela L, et al. Young people’s sexual health in South Africa: HIV prevalence and sexual behaviors from a nationally representative household survey. AIDS 2005; 19:1525–1534. [DOI] [PubMed] [Google Scholar]
- 11.Kaiser R, Bunnell R, Hightower A, Kim AA, Cherutich P, Mwangi M, et al. Factors associated with HIV infection in married or cohabitating couples in Kenya: results from a nationally representative study. PLoS One 2011; 6:e17842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biraro S, Ruzagira E, Kamali A, Whitworth J, Grosskurth H, Weiss HA. HIV-1 transmission within marriage in rural Uganda: a longitudinal study. PLoS One 2013; 8:e55060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akullian A, Bershteyn A, Klein D, Vandormael A, Bärnighausen T, Tanser F. Sexual partnership age pairings and risk of HIV acquisition in rural South Africa. AIDS 2017; 31:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harling G, Newell M, Tanser F, Kawachi I, Subramanian S, Bärnighausen T. Do age-disparate relationships drive HIV incidence in young women? Evidence from a population cohort in rural KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr 2014; 66:443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balkus JE, Nair G, Montgomery ET, Mishra A, Palanee-phillips T, Ramjee G, et al. Age-disparate partnerships and risk of HIV-1 acquisition among South African women participating in the VOICE trial. J Acquir Immune Defic Syndr 2015; 70: 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Oliveira T, Kharsany ABM, Gräf T, Cawood C, Khanyile D, Grobler A, et al. Transmission networks and risk of HIV infection in KwaZulu-Natal, South Africa: a community-wide phylogenetic study. Lancet HIV 2016; 3018:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaefer R, Gregson S, Eaton JW, Mugurungi O, Rhead R, Takaruza A, et al. Age-disparate relationships and HIV incidence in adolescent girls and young women: evidence from a general-population cohort in Zimbabwe. AIDS 2017; 31:1461–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pettifor A, MacPhail C, Selin A, Gomez-Olive FX, Rosenberg M, Wagner RG, et al. HPTN 068: a randomized control trial of a conditional cash transfer to reduce HIV infection in young women in South Africa: study design and baseline results. AIDS Behav 2016; 20:1863–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pettifor A, MacPhail C, Hughes JP, Selin A, Wang J, Gómez-Olivé FX, et al. The effect of a conditional cash transfer on HIV incidence in young women in rural South Africa (HPTN 068): a phase 3, randomised controlled trial. Lancet Glob Health 2016; 4:e978–e988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahn K, Collinson MA, Xavier Gómez-olivé F, Mokoena O, Twine R, Mee P, et al. Profile: Agincourt health and socio-demographic surveillance system. Int J Epidemiol 2012; 41:988–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delany-Moretlwe S, Jentsch U, Weiss H, Moyes J, Ashley-Morrow R, Stevens W, et al. Comparison of focus HerpesSelect and Kalon HSV-2 gG2 ELISA serological assays to detect herpes simplex virus type 2 antibodies in a South African population. Sex Transm Infect 2009; 86:46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cluver L, Gardner F, Operario D. Psychological distress amongst AIDS-orphaned children in urban South Africa. J Child Psychol Psychiatry 2007; 48:755–763. [DOI] [PubMed] [Google Scholar]
- 23.Smit J, Myer L, Middelkoop K, Seedat S, Wood R, Bekker LG, et al. Mental health and sexual risk behaviours in a South African township: a community-based cross-sectional study. Public Health 2006; 120:534–542. [DOI] [PubMed] [Google Scholar]
- 24.Pulerwitz J, Gortmaker SL, Dejong W. Measuring sexual relationship power in HIV/STD research. Sex Roles 2000; 42:637–660. [Google Scholar]
- 25.Boyes ME, Cluver LD. Performance of the Revised Children’s Manifest Anxiety Scale in a sample of children and adolescents from poor urban communities in Cape Town. Eur J Psychol Assess 2013; 29:113–120. [Google Scholar]
- 26.Jewkes RK, Dunkle K, Nduna M, Shai N. Intimate partner violence, relationship power inequity, and incidence of HIV infection in young women in South Africa: a cohort study. Lancet 2010; 376:41–48. [DOI] [PubMed] [Google Scholar]
- 27.Prakash A, Risser RC, Mallinckrodt CH. The impact of analytic method on interpretation of outcomes in longitudinal clinical trials. Int J Clin Pract 2008; 62:1147–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma Hernán, Brumback B Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000; 11:561–570. [DOI] [PubMed] [Google Scholar]
- 29.Cole SR, Hudgens MG. Survival analysis in infectious disease research: describing events in time. AIDS 2010; 24:2423–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu S, Shetterly S, Powers D, Raebel MA, Tsai TT, Ho PM, et al. Extension of Kaplan-Meier methods in observational studies with time-varying treatment. Value Heal 2012; 15:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie J, Liu C. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med 2005; 24:3089–3110. [DOI] [PubMed] [Google Scholar]
- 32.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed 2004; 75:45–49. [DOI] [PubMed] [Google Scholar]
- 33.Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med 2000; 19:1141–1164. [DOI] [PubMed] [Google Scholar]
- 34.Efron B The efficiency of Cox’s likelihood function for censored data. J Am Stat Assoc 1977; 72:557–565. [Google Scholar]
- 35.UCLA, Institute for Digital Research, Education. Testing the proportional hazard assumption in Cox models. 2018. https://stats.idre.ucla.edu/other/examples/asa2/testing-the-proportional-hazard-assumption-in-cox-models/. [Accessed January 2018]
- 36.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008; 168: 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Agostino RB, Lee ML, Belanger a J, Cupples La, Anderson K, Kannel WB. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat Med 1990; 9:1501–1515. [DOI] [PubMed] [Google Scholar]
- 38.Cole SR, Hernán MA, Robins JM, Anastos K, Chmiel J, Detels R, et al. Effect of highly active antiretroviral therapy on time to acquired immunodeficiency syndrome or death using marginal structural models. Am J Epidemiol 2003; 158:687–694. [DOI] [PubMed] [Google Scholar]
- 39.Naimi AI, Moodie EEM, Auger N, Kaufman JS. Constructing inverse probability weights for continuous exposures: a comparison of methods. Epidemiology 2014; 25:292–299. [DOI] [PubMed] [Google Scholar]
- 40.Robins JM, Hernán Ma, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000; 11:550–560. [DOI] [PubMed] [Google Scholar]
- 41.Evans M, Risher K, Zungu N, Shisana O, Moyo S, Celentano DD, et al. Age-disparate sex and HIV risk for young women from 2002 to 2012 in South Africa. J Int AIDS Soc 2016; 19:21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans M, Maughan-Brown B, Zungu N, George G. HIV prevalence and ART use among men in partnerships with 15–29 year old women in South Africa: HIV risk implications for young women in age-disparate partnerships. AIDS Behav 2017; 21:2533–2542. [DOI] [PubMed] [Google Scholar]
- 43.Morrison-Beedy D, Carey MP, Tu X. Accuracy of audio computer-assisted self-interviewing (ACASI) and self-administered questionnaires for the assessment of sexual behavior. AIDS Behav 2006; 10:541–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houle B, Angotti N, Clark SJ, Williams J, Gómez-Olivé FX, Menken J, et al. Let’s talk about sex, maybe: interviewers, respondents, and sexual behavior reporting in rural South Africa. Field Methods 2014; 28:112–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stoner M, Edwards J, Miller W, Halpern CT, Aiello A, Julien A, et al. The effect of schooling on partner age difference and number of sexual partners among young women in rural South Africa enrolled in HPTN 068. J Acquir Immune Defic Syndr 2017; 76:e107–e114. [DOI] [PMC free article] [PubMed] [Google Scholar]