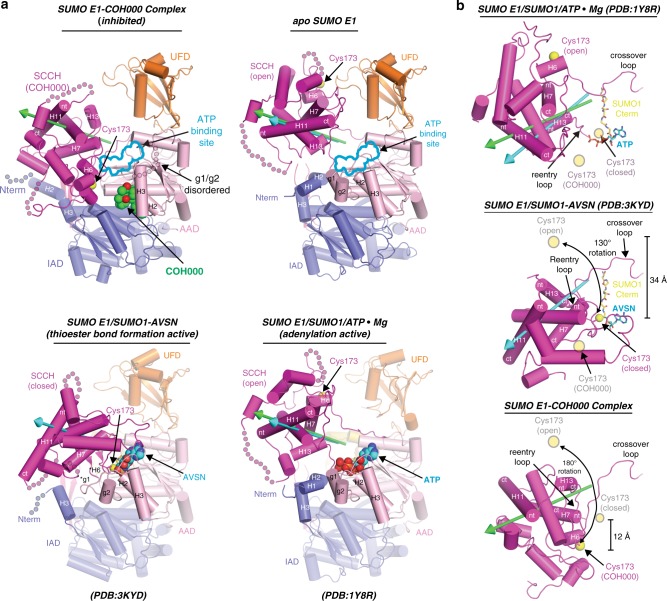
Fig. 1.
Overall structure of SUMO E1COH000 and comparison to other SUMO E1 conformational snapshots. a Comparison of the SUMO E1COH000, SUMO E1APO, SUMO E1/SUMO1/ATP•Mg (PDB: 1Y8R), and SUMO E1/SUMO1-AVSN (PDB: 3KYD) structures, shown as cartoon representations. The green and cyan arrows highlight the rotation axes during transition of the SCCH domain from open to COH000-inhibited and open to closed conformations, respectively. AVSN is an adenosine analog harboring an electrophilic vinyl sulfonamide that was used to covalently trap the tetrahedral intermediate generated during SUMO E1–SUMO thioester bond formation27,39. Asterisks indicate helices that undergo structural remodeling in the thioester bond formation active structure. SUMO E1 domains are labeled and color-coded. COH000, ATP, AVSN, and the SUMO E1 catalytic cysteine are shown as spheres. Selected helices of the SCCH domain are labeled and their N and C termini are indicated by ‘nt’ and ‘ct’, respectively. The ATP-binding sites of the SUMO E1COH000 and SUMO E1APO structures are indicated with a cyan outline. Regions of disorder are indicated with semitransparent circles. b Comparison of SUMO E1 SCCH domain in the open (adenylation active), closed (thioester bond formation active), and COH000 (inhibited) conformations. The adenylation domains (which serve as the rigid body of SUMO E1) were superimposed and the SCCH domains are shown as cartoons with the catalytic cysteines shown as spheres. SCCH domain alternations are highlighted by double-headed arrows and the degree of rotation between each conformational state (with reference to the open conformation) is indicated. Rotation axes of the SCCH domains are highlighted as in a. To provide a frame of reference, the relative position of the catalytic cysteine in the other SCCH domain conformational states are indicated with semitransparent yellow circles and labeled accordingly in each of the panels