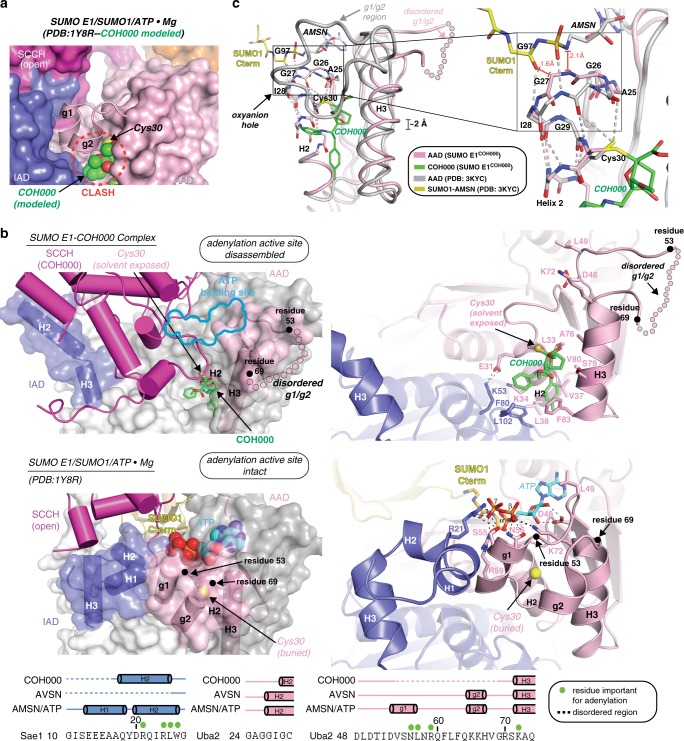
Fig. 3.
Small- and large-scale conformational changes are induced in SUMO E1 upon COH000 binding. a COH000 (spheres) was docked onto SUMO E1 from the SUMO E1/SUMO1/ATP•Mg structure (PDB: 1Y8R). SUMO E1 is shown as a surface representation with the exception of the g1/g2 region, which is shown as cartoon. The steric clash between COH000 and the g1/g2 region is highlighted by a dashed red circle. Two views of the structure, rotated by 90° about the y-axis are presented. b The SUMO E1COH000 (top) and SUMO E1/SUMO1/ATP•Mg (PDB: 1Y8R; middle) structures are shown in the same orientation to highlight conformational changes that occur upon COH000 binding. Regions undergoing conformational changes are colored by their domain as in Fig. 1a and the rest of the structure is colored light gray. For clarity, the SCCH domains are not shown in the magnified views presented in the right panels. b (bottom) Structure-based sequence alignment of SUMO E1 active site regions undergoing conformational changes upon COH000 binding. Secondary structure for the indicated structures are shown above the sequence colored with dashed lines indicating disorder. Residues important for catalysis of adenylation are highlighted by green circles. c (left) The adenylation domains of the SUMO E1COH000 and SUMO E1/SUMO1-AMSN (PDB: 3KYC) structures were superimposed and are shown as colored light pink and gray tubes, respectively. AMSN is an adenosine analog harboring a sulfamide group that was used to generate a nonhydrolyzable mimic of the SUMO1-adenylate intermediate27,39. The N-terminal half of AAD H2 is shown as sticks and the oxyanion hole is boxed. The 2 Å shift of H3 induced upon COH000 binding is indicated and the disordered g1/g2 region of the SUMO E1COH000 structure is shown as semitransparent light pink circles. The C terminus of SUMO1 (yellow) and AMSN (gray) are shown as sticks. c (right) Magnified view around the SUMO E1 oxyanion hole