Fig. 4.
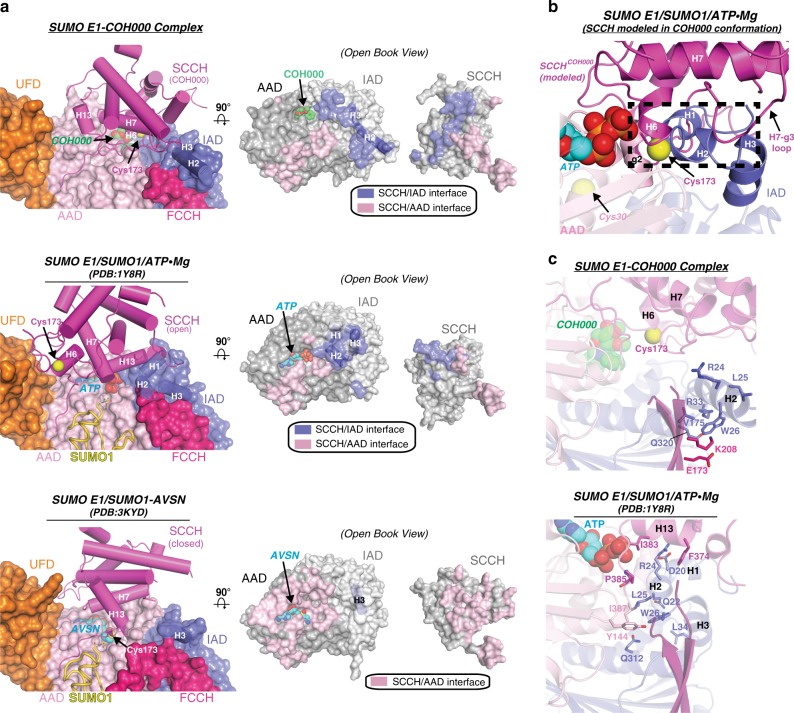
COH000 exploits allosteric coupling of the SUMO E1 adenylation and SCCH domains. a (left) The adenylation domains and UFD of the indicated structures are shown as surface representations and the SCCH domains are shown as cartoons. The SUMO E1 catalytic cysteines, COH000, ATP, and AVSN are shown as spheres. a (right) Open book representation of the indicated structures shown in a top-down view relative to the left panel. Residues buried at the IAD/SCCH and AAD/SCCH interfaces are colored slate and pink, respectively. b The SCCH domain is modeled onto an adenylation active SUMO E1COH000 structure in the COH000-inhibited conformation. The model was created by superimposing the adenylation domains of the SUMO E1COH000 and SUMO E1/SUMO1/ATP•Mg (PDB: 1Y8R) structures. The SUMO E1/SUMO1/ATP•Mg structure is shown as cartoon, and the SCCH domain is not shown for clarity. Only the SCCH domain from the SUMO E1COH000 structure is presented (as a cartoon). The SUMO E1 catalytic cysteine (Cys173), Cys30, and ATP are shown as spheres. Regions involved in steric clashes between the adenylation domains and the SCCH domain modeled in the COH000 conformation are highlighted with a dashed black box. c The indicated structures are shown as cartoon representations. The RLW motif of the Sae1 IAD and SUMO E1 residues involved in contacts to the RLW motif are shown as sticks