Abstract
Increasing incubation temperatures may threaten the viability of sea turtle populations. We explored opportunities for decreasing incubation temperatures at a Caribbean rookery with extreme female-biased hatchling production. To investigate the effect of artificial shading, temperatures were measured under simple materials (white sheet, white sand, palm leaves). To test natural drivers of incubation temperature, temperatures were measured at average nest depths with shading on two beaches. Results from a pilot experiment suggest the most effective material was palm leaves. Shading decreased temperatures by a mean of 0.60 °C (SE = 0.10 °C, N = 20). Variation between beaches averaged 1.88 °C (SE = 0.13 °C, N = 20). We used long-term rookery data combined with experimental data to estimate the effect on sex ratio: relocation and shading could shift ratios from current ranges (97–100% female) to 60–90% female. A conservation mitigation matrix summarises our evidence that artificial shading and nest relocation are effective, low-cost, low-technology conservation strategies to mitigate impacts of climate warming for sea turtles.
Introduction
Climate change continues at unprecedented rates with well-documented impacts across terrestrial and marine environments, primarily affecting ecosystem function, species abundance and distributions1. Climate change models indicate an increase in temperature, sea level and precipitation2. These predictions are highly relevant to oviparous reptiles due to direct ecological threats affecting nest flooding, nesting site availability and suboptimal sex allocation in species with environmental sex determination3. Furthermore, population-scale consequences associated with increased temperatures arise in taxa exhibiting temperature-dependent sex determination (TSD), such as reptiles and some species of fish4. Hence understanding how climate change will impact sex ratios and population viability in sea turtles, and how these impacts may be mitigated, have been identified as key issues5.
Sea turtles exhibit TSD with female hatchlings produced at higher temperatures, males at cooler temperatures and a balanced sex ratio at the pivotal temperature (around 29 °C)6. Higher temperatures not only increase female-biased sex ratios but also increase egg mortality, with these effects being consistent across populations and species7,8. With the average global temperature predicted to increase by at least 2.6 °C by 21009, warming temperatures threaten sea turtles through effects on hatching sex ratio skews and increased hatchling mortality10.
While there has been long-term concern over the status of sea turtles, conservation efforts around the world have led to increases in nesting numbers for a wide range of populations and species11. Yet climate warming remains a threat for the viability of this group broadly and so assessing the effects of climate change was identified as a top global research priority for successful future conservation of turtles5 and quantification of the effect of warming temperatures is a conservation priority. Recent research efforts have focused on current and predicted hatchling sex ratios7 with concerns that high female-biased primary sex ratios are already common at most sea turtle rookeries, exceeding 3:1 in >50% studies12. Many sea turtle rookeries are producing female-biased populations (e.g., Brazil13, Caribbean14,15, Florida16, Mediterranean17, Australia8), or will skew towards near feminisation of hatchling output within 50 years (e.g. Australia18).
There are relatively few sea turtle rookeries where cooler sand temperatures produce balanced hatchling sex ratios. Geographic locations at the latitudinal extreme of nesting ranges is an obvious driver of cooler temperatures, for example South Brazil and North Carolina in the Atlantic10,19. Natural beach variability and vegetation shading are important drivers for cooler temperatures on a remote Indian Ocean atoll20. Other studies have shown that coastal vegetation shading can offset or delay climate change driven female-biased primary sex ratios, e.g., Guadeloupe, Caribbean21. However, for sea turtle rookeries without coastal vegetation (e.g. Great Barrier Reef islands in Australia, Ascension Island and some Cape Verdean islands in the Atlantic), the restricted natural variation in beach temperatures provides little or no resilience for a balanced primary sex ratio in future. This has led to the development of management interventions to conserve turtle populations, and options for decreasing incubation temperatures include artificial shading22, watering and relocation to greater sand depths23. While the general impact of shading, increased depth and watering to cool nests is well known, it is not known if the magnitude of these impacts is invariant across sites and hence whether they will provide the same impact as a management intervention across the world. Hence further trials of methods to artificially cool nests are needed.
The present study explores opportunities for decreasing incubation temperatures at a rookery with extreme female-biased hatchling production in St Eustatius, North East Caribbean. Recent incubation temperature studies suggested that three species nesting at this rookery (leatherback turtle, Dermochelys coriacea, hawksbill turtle, Eretmochelys imbricata and green turtle, Chelonia mydas) have had female-biased hatchling production for at least a century with <36% males produced every year14. In this study, our aim was to examine the thermal effect of different shading techniques and consequent hatchling sex ratio. (1) We investigate the most efficient low-technology, low-cost shading technique. (2) Informed by the results of shading experimentation, we examine the effect of environmental variables (beach, shade, depth) on temperature and hatchling sex ratio. (3) Using the results from the effect of different environmental variables, predict potential benefits of conservation actions and likely consequence for current and future primary sex ratios at a rookery with extreme female bias.
Results
Assessment of shading techniques
Data were successfully retrieved from the six loggers on Zeelandia Beach. All loggers collected data for a period of 69 hours.
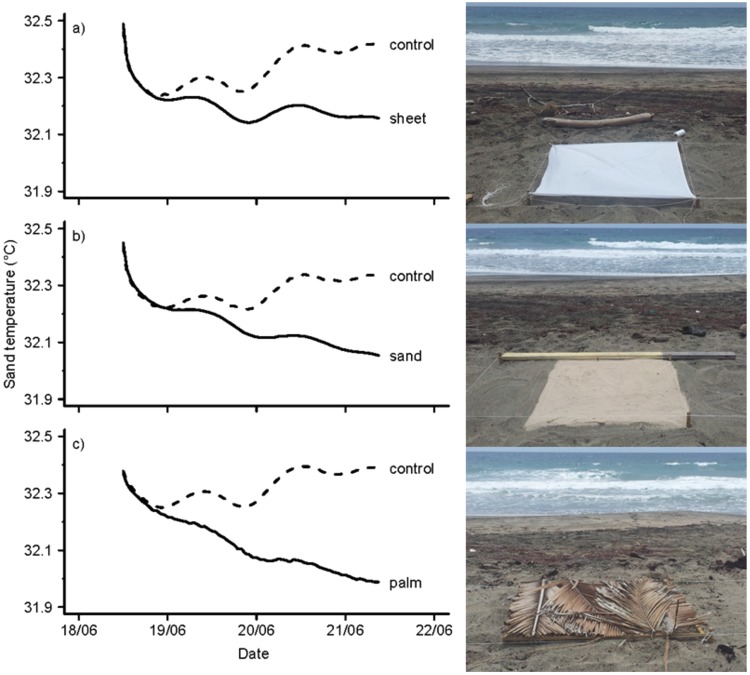
All three treatments were effective at cooling sand temperatures (Fig. 1). At the end of the experiment (i.e. after 69 hours) sand temperature recorded under the white cotton sheet was 0.26 °C lower than the corresponding control temperature. Temperature recorded under the white sand was 0.28 °C lower than its corresponding control temperature, and temperature under the palm leaves was 0.40 °C lower than its corresponding control temperature.
Figure 1.
Three different shading techniques were used to cool sand temperatures at mean hawksbill and green turtle nest depth (50 cm). The maximum difference between sand temperatures recorded under the white cotton sheet (a) and corresponding control temperatures was 0.26 °C. The maximum difference between sand temperatures recorded under the white sand (b) and control temperatures was 0.28 °C. The maximum difference between sand temperatures recorded under the palm leaves (c) and control temperatures was 0.40 °C. This pilot experiment ran from 18 June 2012 until 21 June 2012 and provides a conservative estimate as the temperature differences between controls and shading treatments were expected to increase further after these 3 days.
To quantify the effect of shading on temperature we calculated the difference between sand temperatures recorded in a treatment plot (i.e. shaded with white cotton sheet, white sand or palm leaves) and sand temperatures recorded in the associated control plot. To reduce the effect of temporal auto-correlation while retaining enough points for analysis we used 5-hour means in the analysis. An analysis of covariance confirms that treatment had an effect on sand temperature (F5,36 = 367.3, p < 0.05, 95% Confidence Interval (CI) = −0.0355, −0.0308). While all three treatments successfully lowered sand temperatures, palm leaves proved to be the most effective treatment, reducing sand temperatures by approximately 0.3 °C during the first 50 hours of the experiment (F1,12 = 498.6, p < 0.05, CI = −0.0363, −0.0299). Using white sand as a treatment reduced sand temperatures by approximately 0.2 °C during the first 50 hours of the experiment (F1,12 = 476.7, p < 0.05, CI = −0.0268, −0.0219). Using the white cotton sheet also reduced sand temperatures by approximately 0.2 °C during the first 50 hours of the experiment (F1,12 = 918.6, p < 0.05, CI = −0.0235, −0.0203). At the end of 3 days, the difference between temperatures under treatment plots and temperatures under the corresponding control plots continued to show a linear and decreasing trend. Realistically, these differences should gradually reach horizontal asymptotes. Unfortunately our experiment did not run long enough to reveal this. Regardless, the results from this short pilot experiment informed with confidence which treatment was most effective at cooling sand temperatures (see Fig. 1).
Effect of depth, shade and beach on sand temperature
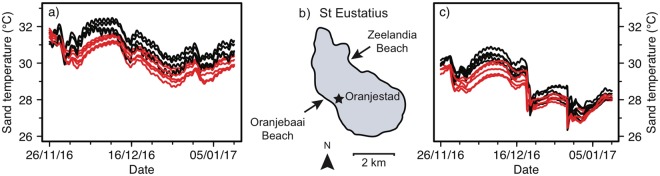
Four loggers were lost due to beach erosion. The remaining 20 loggers were successfully retrieved and included in the analysis. Data from 26 November 2016 until 10 January 2017 were used for the analyses, covering a 46-day period (Fig. 2).
Figure 2.
Sand temperatures recorded on St Eustatius between 26 November 2016 and 10 January 2017. A total of 20 temperature loggers recorded sand temperatures on (a) Oranjebaai Beach (n = 4 for control, n = 6 treatment) and (c) Zeelandia Beach (n = 5 for control, n = 5 treatment) in control plots (black lines) and plots shaded by palm leaves (red lines) at mean turtle nest depth (50 and 63 cm).
To study the relationship between sand temperature, depth (i.e. 50 vs 63 cm), treatment (i.e. control vs shaded) and beach (i.e. Oranjebaai Beach vs Zeelandia Beach) mean sand temperatures were calculated for each logger. We performed a linear mixed model analysis to understand the relationship between sand temperature, depth, treatment and beach. Depth, treatment and beach were entered as fixed effects. Site and plot were entered as random effects. Normality of the residuals was checked through visual inspection of the residual plots. Likelihood Ratio Tests were used to obtain P-values. We tested the significance of the three-way and all two-way interactions by comparing a model with no interactions to a model with one interaction of interest.
The main effect of beach on sand temperature was clearly visible in our data, with sand temperatures on Oranjebaai Beach being approximately 2.0 °C higher than sand temperatures on Zeelandia Beach (Figs 2, 3). Similarly, the effect of treatment (control vs shaded) is clear, with shaded plots being approximately 0.6 °C cooler than control plots (Figs 2, 3). In contrast, depth visibly shows no effect on sand temperatures (Fig. 3). The effect of shading increased by a factor of 2 (from approximately 0.3 °C to 0.6 °C) and results from the earlier shorter experiment are a conservative estimate. None of the interactions were significant (χ2 = 5.27, df = 4, p = 0.26) so we used a model with no interactions. Our model confirms that beach had a significant effect on sand temperature (χ2 = 21.34, df = 1, p < 0.05, CI = −2.1801, −1.5885). Treatment also had a significant effect on sand temperatures (χ2 = 14.46, df = 1, p < 0.05, CI = −0.8433, −0.3637) while depth did not (χ2 = 1.66, df = 1, p = 0.20).
Figure 3.
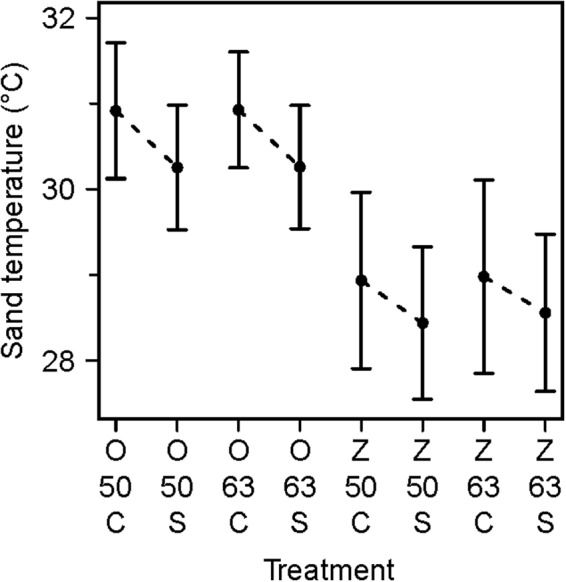
Beach and treatment had an effect on sand temperature. Mean sand temperatures are given for each group of loggers in a treatment plot (i.e. shaded) and associated control plot. The intervals define the standard deviation. O: Oranjebaai Beach; Z: Zeelandia Beach; 50: 50 cm; 63: 63 cm depths; C: Control; S: Shaded. The dashed lines highlight the difference between a control plot and the corresponding shaded plot situated on the same beach and at the same depth.
We constructed a conservation mitigation matrix relating temperature changes between study beaches and treatments (Table 1). The biggest temperature difference was found between control plots on Oranjebaai Beach and shaded plots on Zeelandia Beach (±2.487 °C).
Table 1.
Sand temperature variations (between shading treatment and beaches) provide guidelines for potential conservation actions to decrease sea turtle nest incubation temperature.
| New location | |||||
|---|---|---|---|---|---|
| X | O C | O S | Z C | Z S | |
| Source location | O C | X | −0.60 °C (±0.10 °C) |
−1.88 °C (±0.13 °C) |
−2.48 °C
(±0.23 °C) |
| O S | 0.60 °C (±0.10 °C) |
X | −1.28 °C (±0.23 °C) |
−1.88 °C (±0.13 °C) |
|
| Z C | 1.88 °C (±0.13 °C) |
1.28 °C (±0.23 °C) |
X | −0.60 °C (±0.10 °C) |
|
| Z S | 2.48 °C (±0.23 °C) |
1.88 °C (±0.13 °C) |
0.60 °C (±0.10 °C) |
X | |
Sand temperatures at Zeelandia Beach vary from 31.9–33.3 °C in peak nesting season14. Values represent the difference between a source location (rows) and a new location (columns). Column and row headings represent beach location (O = Oranjebaai Beach; Z = Zeelandia Beach) and treatment (S = shading by palm leaves; C = control). Values in brackets are the standard errors (n = 20).
Primary sex ratios
Mean monthly sand temperatures on Zeelandia Beach are reported to vary from 29.1–29.6 °C in January–March to 31.9–33.3 °C in June–August14. Using the equation describing the relationship between primary sex ratio and incubation temperature7, it is possible to estimate that the primary sex ratios are currently approximately 50–56% female-biased in January–March and 97–100% female-biased in June–August.
Lowering sand temperatures through the implementation of mitigation strategies such as relocating eggs from Oranjebaai Beach to Zeelandia Beach and shading turtle nests would have an effect on sex ratios (Table 2). For example, a change of 1.0 °C would result in primary sex ratios being approximately 21–34% female-biased during January–March and 91–98% female-biased in June–August. A decrease of 2.5 °C would result in primary sex ratios being approximately 4–7% female-biased during January–March and 60–90% female-biased in June–August.
Table 2.
Current and predicted primary sex ratios indicate the potential benefits of implementing conservation mitigation strategies.
| Temperature change (°C) | ||||
|---|---|---|---|---|
| Current | −1 | −2 | −2.5 | |
| January–March | 50–56% | 21–34% | 7–12% | 4–7% |
| June–August | 97–100% | 91–98% | 74–95% | 60–90% |
| 2090 | −1 °C | −2 °C | −2.5 °C | |
| January–March | 98–99% | 95–97% | 83–90% | 71–83% |
| June–August | 100% | 100% | 99–100% | 99–100% |
Current values are based on incubation temperatures recorded on St Eustatius in 2011-201214 calculated using the relationship between incubation temperature and primary sex ratio7. 2090 values are based on incubation temperatures projected for the year 209014. Two seasonal periods are provided: June–August are peak nesting months; occasional nesting by hawksbills and leatherbacks occurs during January–March (JB, NE, unpublished data). All values are given as female percentages (e.g. 60% indicates 60% females and 40% males in a clutch).
It is projected that mean incubation temperatures on St Eustatius will increase by approximately 1.1 °C by the year 2030, by 2.0 °C by 2060 and by 3.2 °C by 209014. Without mitigation strategies, this will result in primary sex ratios being close to 100% female by the end of the century. Nest relocation or artificial shading would only have a measurable effect on primary sex ratios during January–March (Table 2).
Discussion
Our results demonstrate that simple, low cost, low technology artificial shading can have a significant impact on sea turtle incubation conditions. All shading strategies reduced temperature and the most effective treatment was palm leaves, supporting results of previous studies24–26. This contrasts to a previous shading experiment (using solar wave fabric) that had variable results27. Furthermore, we report that relocation to a cooler windward beach can reduce the temperature of the eggs by a factor of four. We calculated the effects of these simple conservation actions, which would result in a positive shift from a female-skewed to a balanced primary sex ratio in the current conditions. The application of our results to mitigation strategies provides grounds for cautious optimism that simple and low-cost conservation actions can be effective at rookeries with high incubation temperatures and female-skewed primary sex ratios.
The effects in temperature decrease due to artificial shading at our study site are comparable to the magnitude of effects of shading by coastal vegetation and forest border on naturally shaded nesting beaches20,21. This suggests that artificial shading can be a substitute for natural vegetation shading at sea turtle rookeries in arid environments (e.g. Great Barrier Reef islands). Depth does not have such a clear effect as shading and differs due to a delayed time-lag response during periods of cooling and warming as well as a reduced pattern of diel variation at depth14,20. Relocation of nests to different depths that reflect depths of natural nests at the same beach may not have the anticipated results because of complicated interactions between depth and the environment. However, our experiments were not designed to look at the full extent of depth impacts, since we only varied the range of depths fairly minimally (50–63 cm) corresponding to the mean natural depth of sea turtle nests on the island, while the difference between the minimum and maximum depth of incubating eggs on the study beach is likely to have a much greater effect. Although relocation practices can negatively affect egg and hatchling survivorship28, examples of successful relocation conservation programmes exist worldwide29,30. Our results further demonstrate that shading in combination with relocation to a different cooler beach can have much greater benefits for a balanced primary sex ratio.
While results from experiments studying the effect of incubation temperature on emergence success have been inconclusive for St Eustatius14, it is possible to use published relationships to estimate the impact mitigation strategies would have on emergence successes. For example, at an incubation temperature of 32.5 °C, emergence success is likely to be <50%7. Lowering temperatures by 2 °C through mitigation strategies (e.g. relocation) could increase the emergence success to >80%. A further 0.5 °C reduction in incubation temperature (e.g. shading) would bring emergence successes close to 85%. This serves to illustrate the impact mitigation strategies can have on both primary sex ratios and emergence successes of sea turtles.
The variation in thermal ecology of two nesting beaches (<1 km distance) at our study site was surprising as the sand on both beaches is of similar (volcanic) origin and within-beach variation was not significant in a previous study14. The strong northeastern trade winds31 are likely to provide a cooling effect for Zeelandia Beach. At this cooler beach, temperatures are low enough to produce a balanced primary sex ratio during off-peak nesting season in current conditions. There is evidence that turtles select particular micro-habitats for nesting. For example, hawksbill turtles nest under vegetation32, loggerheads Caretta caretta nest on mangrove islands33 and painted turtles Chrysemys picta seek shaded nest locations at warmer sites34. Protection of rookeries that appear minor in terms of numbers may be essential for the health of the larger regional population19. Similarly, beaches with a range of shade cover should be identified to allow plasticity in nest-site choice21. It is important to take a regional approach to assess temperature at all rookeries and identify high priority cool beaches for protection.
When projecting into the future, warming air temperatures are predicted to cause the mean incubation temperatures at our study site to reach 32.1 °C by 2030 and 34.2 °C by 209014, above the upper thermal limit of 33 °C for successful incubation35. Alongside warming air temperatures, global sea level rise will inundate beaches and limit available nesting habitat36. In the meantime, these temperatures will exacerbate existing female bias: female green turtle sex ratios have been >95% since 2009 and female sex ratios of leatherbacks and hawksbills are projected to reach >95% by the years 2028 and 2045 respectively14. A key issue remains whether phenological shifts in the seasonal timing of nesting will help mitigate predicted impacts of climate warming. In the absence of phenological shifts in nesting or conservation actions, it is possible that this rookery will no longer support viable nesting turtle populations. However, we urge caution with extending these conclusions to other areas since our results are from a rookery with extreme sand temperatures. Relocating and shading of nests at other rookeries is likely to produce different results than those reported in this study, so we emphasize that our results should be perceived as guidelines only. A vulnerability assessment framework37, microclimate model38 or sensitivity map39 may assist with gaining a thorough understanding of natural variability of beach temperatures before planning conservation strategies such as relocation or artificial shading. A further issue for consideration is the practicality of management interventions, i.e. how to scale up cooling treatments of a few days for a handful of sites up to 100 s or 1000 s of nests over several months.
With incubation temperatures projected to rise by up to 4 °C over the next century at this study site14, these mitigation strategies may prove essential to guarantee that future operational sex ratios and emergence successes are viable. However, the maximum temperature decrease achievable by relocation and artificial shading is less than this predicted temperature increase, so will only be sufficient to maintain a balanced primary sex ratio in the cooler off-peak nesting seasons. Our model is limited by the use of estimated values for primary sex ratio and emergence success. As shown for other rookeries, it is possible that male embryo survival and different male and female breeding periodicities will mitigate the highly female-skewed primary sex ratio7,12. In light of new research that demonstrates that higher temperatures increase the natural growth rate of turtle populations (as more females are produced) but that long-term population survival is threatened (once incubation temperatures near lethal levels), an improved understanding of emergence success may be more of a priority than sex ratios40. Finally, if sex ratios of a small and endangered population are significantly skewed, there may be consideration of a captive breeding programme (e.g. a hatchery) to release additional individuals into the wild to support small or declining populations41 or an artificial change in the conditions at the egg-laying site42. The sudden drops in temperature we attributed to rainfall have also been noted elsewhere on nesting beaches43. This impact of rain on nest temperatures highlights how the impacts of changing rainfall patterns also need to be considered when assessing the impact of climate change on sea turtle hatchling sex ratios.
Effective transboundary conservation of migratory species is a difficult challenge faced by many conservation bodies44. Management of nesting beaches is a widespread practice for endangered turtle populations45. Although results from our study contribute to a greater understanding of conservation strategies to decrease incubation temperature, there is a consensus that greater understanding of risks and effectiveness associated with conservation programmes is required5. Several regional sea turtle conservation networks exist, e.g., Wider Caribbean Sea Turtle Network (WIDECAST; www.widecast.org) and the Indian Ocean–Southeast Asian Memorandum of Understanding for the Conservation and Management of marine turtles and their habitats (www.ioseaturtles.org). These networks may serve as starting points to identify high priority cool beaches for protection and for discussions about a standardised framework for beach conservation actions at rookeries with extreme female-biased primary sex ratios and that lack natural coastal vegetation to enhance natural resilience in the face of global climate change46.
Methods
Study site
The 21 km2 island of St Eustatius is located in the Lesser Antilles in the north-eastern Caribbean. Almost all clutches are laid by leatherback, hawksbill and green turtles on two beaches: Oranjebaai Beach (sheltered, western coast; 17.483°N, 62.988°W) and Zeelandia Beach (exposed, eastern coast; 17.507°N, 62.980°W), the latter being the most important nesting beach46. The typical nesting season is March until June (leatherback), June until November (hawksbill) and July until October (green) (JB, NE, STENAPA unpublished data). The study was conducted within the Statia National Marine Park programme and complied with all relevant local and national legislation.
Temperature loggers
Tinytag Plus 2 loggers (Tinytag Plus 2 model TGP-4017, Gemini Data Loggers, UK) were used to record sand temperature at depths representative of nests for leatherback, hawksbill and green turtles nesting on Oranjebaai Beach and Zeelandia Beach during 2012-2013 and 2016-2017. Temperature measurements were recorded every hour. The loggers were originally calibrated to United Kingdom Accreditation Service (UKAS) standards and are accurate to <0.5 °C (www.tinytag.info, last accessed on 28 March 2018). To minimize impact on natural conditions during burial of loggers, care was taken to excavate a sand core and then replace it back on top of the logger. This was achieved by hammering a 10 cm diameter PVC pipe to the desired depth of the logger, creating a vacuum and then removing the pipe full of sand. The depth of the hole was verified with a semi-rigid tape measure, then the logger was dropped into the hole and the sand was emptied out of the pipe on top of the logger. A string was connected to the logger to facilitate relocation of the loggers. GPS positions of the loggers were recorded. After burial, one day was allowed for the sand temperatures to return to original state after disturbance. To ensure all loggers were exposed to the same degree, the surface of the experimental area was cleared from seaweed patches, other organic material and debris.
Assessment of shading techniques
An artificial shading experiment to examine the effects of low-cost and available shade materials on sand temperatures was conducted over a 72 hour period from 18–22 June 2012 on the principal turtle nesting beach, Zeelandia Beach. Loggers were deployed at mean nest depth of 50 cm (hawksbill and green turtles; JB, NE, STENAPA unpublished data). Three shading materials were used: white cotton sheet, white (imported) sand and (local) palm leaves (Cocos nucifera) (Fig. 1). An area of 1.5 × 10 m was cleared (i.e. organic material and debris were removed) and the sand surface was raked flat. Six loggers were buried in a row parallel to the waterline at 1.5 m intervals and 50 cm depth. After burying loggers, artificial shading methods (1 m² surface area) were immediately placed on the sand surface so that each logger was centrally located within the experimental plot.
Effect of depth, shade and beach on sand temperature
Loggers were buried at mean nest depths of 50 cm (hawksbill and green turtles) and 63 cm (leatherback turtles). Mean depths were calculated from records as the midpoint between the top and bottom of clutches of eggs excavated between 2005–2015 (JB, NE, STENAPA unpublished data). To examine the key drivers of sand temperature we measured the impact of shading and nest depth at two beaches: Oranjebaai Beach (Southwest coast) and Zeelandia Beach (Northeast coast). At each beach three different plots were selected as replicates. In total, 24 loggers were buried: 12 were buried at each site (beach); at each site 4 were buried in each plot (replicates); within each plot 2 were buried for each treatment (control vs shaded); within each treatment 1 logger was buried at each depth (50 or 63 cm). The experiments were conducted from 23/09/2016–22/11/2016 and from 25/11/2016 until 09/01/2017. Initially the Oranjebaai Beach experiment was at the north end. A heavy storm on 17/11/2016 eroded the beach, several loggers were lost and loggers were relocated (using the same treatments) to the south end of Oranjebaai Beach. Shaded areas were created using palm leaves attached to 1 m² wooden frames with biodegradable cotton string and placed over the buried loggers. The wooden frames were made using branches from small native trees. The palm leaves were collected from the ground next to palm trees around St Eustatius.
Data were downloaded from temperature loggers using TinyTag Explorer 4.7. Prior to the analyses, data from before logger deployments, during relocation periods, and from after retrievals were discarded. All datasets were reviewed and checked for anomalies. We used R47 and package lme448 for analyses.
Primary sex ratios
The relationship between incubation temperature and primary sex ratio7 was used to estimate the potential that mitigation strategies have to affect sex ratios at this field site. We used this model to calculate the expected change in primary sex ratios with cooling regimes of 1 °C, 2 °C and 2.5 °C.
Acknowledgements
We thank students and volunteers that supported S.U., F.S.P.L.K. and J.B. during experimental work. Experimental design fieldwork by N.E. was supported by IMARES. M.J.A.C. and L.B. were supported through the project “Ecology and conservation of green and hawksbill turtles in the Dutch Caribbean” funded by the Netherlands Organization of Scientific Research (NWO-ALW 858.14.090). Fieldwork of F.S.P.L.K. was supported by the FONA foundation and Alberta Mennega Stichting. The authors acknowledge the use of the Maptool program (www.seaturtle.org) for the production of Figure 2.
Author Contributions
N.E. and M.J.A.C. conceived the study. N.E., E.H.M. and S.U. developed the experimental shading design and S.U. carried out fieldwork. N.E., M.J.A.C., L.E.B. and F.S.P.L.K. developed the experimental design to test drivers of temperature and F.S.P.L.K. conducted fieldwork. J.B. supported all fieldwork. J.-O.L. led the data analysis with assistance from N.E. and G.C.H. N.E. led the writing of the manuscript with contributions from all authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jones MC, Cheung WWL. Multi-model ensemble projects of climate change effects on global marine biodiversity. ICES J. Mar. Sci. 2015;72:741–752. doi: 10.1093/icesjms/fsu172. [DOI] [Google Scholar]
- 2.Easterling DR, et al. Climate extremes: observations, modelling, and impacts. Science. 2000;289:2068–2074. doi: 10.1126/science.289.5487.2068. [DOI] [PubMed] [Google Scholar]
- 3.Mainwaring MC, et al. Climate change and nesting behaviour in vertebrates: a review of the ecological threats and potential for adaptive responses. Biol. Rev. 2017;92:1991–2002. doi: 10.1111/brv.12317. [DOI] [PubMed] [Google Scholar]
- 4.Somero GN. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 2010;213:912–920. doi: 10.1242/jeb.037473. [DOI] [PubMed] [Google Scholar]
- 5.Rees AF, et al. Are we working towards global research priorities for management and conservation of sea turtles? Endanger. Species Res. 2016;31:337–382. doi: 10.3354/esr00801. [DOI] [Google Scholar]
- 6.Ackerman, R. A. The nest environment and the embryonic development of sea turtles. Pages 83–106 in Lutz, P. L. & Musick, J. A. (editors), The Biology of Sea Turtles, Volume I. CRC Press, Boca Raton, Florida, USA (1997).
- 7.Hays GC, Mazaris AD, Schofield G, Laloë J-O. Population viability at extreme sex-ratio skews produced by temperature-dependent sex determination. Proc. R. Soc. B. 2017;284:20162576. doi: 10.1098/rspb.2016.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen MP, et al. Environmental warming and feminization of one of the largest sea turtle populations in the world. Curr. Biol. 2018;28:154–159. doi: 10.1016/j.cub.2017.11.057. [DOI] [PubMed] [Google Scholar]
- 9.IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, Pachauri, R. K. and Meyer, L. A. (eds)]. IPCC, Geneva, Switzerland, 151 pp (2014).
- 10.Hawkes LA, Broderick AC, Godfrey MH, Godley BJ. Investigating the potential impacts of climate change on a marine turtle population. Global Change Biol. 2007;13:923–932. doi: 10.1111/j.1365-2486.2007.01320.x. [DOI] [Google Scholar]
- 11.Mazaris AD, Schofield G, Gkazinou C, Almpanidou V, Hays GC. Global sea turtle conservation successes. Sci. Adv. 2017;3:e1600730. doi: 10.1126/sciadv.1600730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hays GC, Mazaris AD, Schofield G. Different male vs. female breeding periodicity helps mitigate offspring sex ratio skews in sea turtles. Front. Mar. Sci. 2014;1:43. doi: 10.3389/fmars.2014.00043. [DOI] [Google Scholar]
- 13.Marcovaldi MA, Godfrey MH, Mrosovsky N. Estimating sex ratios of loggerhead turtles in Brazil from pivotal incubation durations. Can. J. Zool. 1997;75:755–770. doi: 10.1139/z97-097. [DOI] [Google Scholar]
- 14.Laloë J-O, Esteban N, Berkel J, Hays GC. Sand temperatures for nesting sea turtles in the Caribbean: Implications for hatchling sex ratios in the face of climate change. J. Exp. Mar. Bio. Ecol. 2016;474:92–99. doi: 10.1016/j.jembe.2015.09.015. [DOI] [Google Scholar]
- 15.Patino‐Martinez J, Marco A, Quiñones L, Hawkes LA. A potential tool to mitigate the impacts of climate change to the Caribbean leatherback sea turtle. Glob. Change Biol. 2012;18:401–411. doi: 10.1111/j.1365-2486.2011.02532.x. [DOI] [Google Scholar]
- 16.Hanson J, Wibbels T, Martin RE. Predicted female bias in sex ratios of hatchling loggerhead sea turtles from a Florida nesting beach. Can. J. Zool. 1998;76:1850–1861. doi: 10.1139/z98-118. [DOI] [Google Scholar]
- 17.Broderick AC, Godley BJ, Reece S, Downie JR. Incubation periods and sex ratios of green turtles: highly female biased hatchling production in the eastern Mediterranean. Mar. Ecol. Prog. Ser. 2000;202:273–281. doi: 10.3354/meps202273. [DOI] [Google Scholar]
- 18.Fuentes MMPB, et al. Proxy indicators of sand temperature help project impacts of global warming on sea turtles in northern Australia. Endanger. Species Res. 2009;9:33–40. doi: 10.3354/esr00224. [DOI] [Google Scholar]
- 19.Baptistotte C, Scalfoni JT, Mrosovsky N. Male-producing thermal ecology of a southern loggerhead turtle nesting beach in Brazil: implications for conservation. Anim. Conserv. 1999;2:9–13. doi: 10.1111/j.1469-1795.1999.tb00043.x. [DOI] [Google Scholar]
- 20.Esteban N, Laloë J-O, Mortimer JA, Hays GC, Guzman A. Male hatchling production in sea turtles from one of the world’s largest marine protected areas, the Chagos Archipelago. Sci. Rep. 2016;6:20339. doi: 10.1038/srep20339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamel SJ. Vegetation cover predicts temperature in nests of the hawksbill sea turtle: implications for beach management and offspring sex ratios. Endanger. Species Res. 2013;20:41–48. doi: 10.3354/esr00489. [DOI] [Google Scholar]
- 22.Klein CJ, et al. Prioritization of marine turtle management projects: a protocol that accounts for threats to different life history stages. Conserv. Lett. 2017;10:547–554. doi: 10.1111/conl.12324. [DOI] [Google Scholar]
- 23.Rivas M. L., Spínola M., Arrieta H., Faife-Cabrera M. Effect of extreme climatic events resulting in prolonged precipitation on the reproductive output of sea turtles. Animal Conservation. 2018;21(5):387–395. doi: 10.1111/acv.12404. [DOI] [Google Scholar]
- 24.Hill JE, Paladino FV, Spotila JR, Tomillo PS. Shading and Watering as a Tool to Mitigate the Impacts of Climate Change in Sea Turtle Nests. PLoS One. 2015;10(6):e0129528. doi: 10.1371/journal.pone.0129528.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood A, Booth DT, Limpus CJ. Sun exposure, nest temperature and loggerhead turtle hatchlings: Implications for beach shading management strategies at sea turtle rookeries. J. Exp. Mar. Biol. Ecol. 2014;451:105–114. doi: 10.1016/j.jembe.2013.11.005. [DOI] [Google Scholar]
- 26.Valenzuela NC. shift, and natural temperature effects on sex determination in Podocnemis expansa turtles. J. Ecol. 2001;82:3010–3024. doi: 10.1890/0012-9658(2001)082[3010:CSANTE]2.0.CO;2. [DOI] [Google Scholar]
- 27.Jourdan J, Fuentes MMPB. Effectiveness of strategies at reducing sand temperature to mitigate potential impacts from changes in environmental temperature on sea turtle reproductive output. Mitig. Adapt. Strateg. Glob. Change. 2013;20:121–133. doi: 10.1007/s11027-013-9482-y. [DOI] [Google Scholar]
- 28.Revuelta O, et al. Assessing the efficacy of direct conservation interventions: clutch protection of the leatherback marine turtle in the Dominican Republic. Oryx. 2015;49:677–686. doi: 10.1017/S0030605313001488. [DOI] [Google Scholar]
- 29.Maulany RI, Booth DT, Baxter GS. Emergence success and sex ratio of natural and relocated nests of olive ridley turtles from Alas Purwo National Park, East Java, Indonesia. Copeia. 2012;2012:738–747. doi: 10.1643/CH-12-088. [DOI] [Google Scholar]
- 30.McElroy ML, Dodd MG, Castleberry SB. Effects of common loggerhead sea turtle nest management methods on hatching and emergence success at Sapelo Island, Georgia, USA. Chelonian Conserv. Biol. 2015;14:49–55. doi: 10.2744/ccab-14-01-49-55.1. [DOI] [Google Scholar]
- 31.Chadee XT, Clarke RM. Large-scale wind energy potential of the Caribbean region using near-surface reanalysis data. Renew. Sust. Energ. Rev. 2014;30:45–58. doi: 10.1016/j.rser.2013.09.018. [DOI] [Google Scholar]
- 32.Kamel SJ, Mrosovsky N. Deforestation: risk of sex ratio distortion in hawksbill sea turtles. Ecol. Appl. 2006;16:923–931. doi: 10.1890/1051-0761(2006)016[0923:DROSRD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Foley AM, Peck SA, Harman GR. Effects of sand characteristics and inundation on the hatching success of loggerhead sea turtle (Caretta caretta) clutches on low-relief mangrove islands in southwest Florida. Chelonian Conserv. Biol. 2006;5:32–41. doi: 10.2744/1071-8443(2006)5[32:EOSCAI]2.0.CO;2. [DOI] [Google Scholar]
- 34.Refsnider JM, Warner DA, Janzen FJ. Does shade cover availability limit nest-site choice in two populations of a turtle with temperature-dependent sex determination? J. Therm. Biol. 2013;38:152–158. doi: 10.1016/j.jtherbio.2013.01.003. [DOI] [Google Scholar]
- 35.Miller, J. D. Reproduction in sea turtles. Pages 65–96 in P. L. Lutz and J. A. Musick (editors), The Biology of Sea Turtles, Volume I. CRC Press, Boca Raton, Florida, USA. (1997).
- 36.Katselidis KA, Schofield G, Stamou G, Dimopoulos P, Pantis JD. Employing sea-level rise scenarios to strategically select sea turtle nesting habitat important for long-term management at a temperate breeding area. J. Exp. Mar. Bio. Ecol. 2014;450:47–54. doi: 10.1016/j.jembe.2013.10.017. [DOI] [Google Scholar]
- 37.Fuentes MMPB, Limpus CJ, Hamann M. Vulnerability of sea turtle nesting grounds to climate change. Global Change Biol. 2011;17:140–153. doi: 10.1111/j.1365-2486.2010.02192.x. [DOI] [Google Scholar]
- 38.Fuentes MMPB, Porter WP. Using a microclimate model to evaluate impacts of climate change on sea turtles. Ecol. Model. 2013;251:150–157. doi: 10.1016/j.ecolmodel.2012.12.020. [DOI] [Google Scholar]
- 39.Lopez GG, et al. Coastal development at sea turtles nesting ground: Efforts to establish a tool for supporting conservation and coastal management in northeastern Brazil. Ocean Coast. Manage. 2015;116:270–276. doi: 10.1016/j.ocecoaman.2015.07.027. [DOI] [Google Scholar]
- 40.Laloë J-O, Cozens J, Renom B, Taxonera A, Hays GC. Climate change and temperature‐linked hatchling mortality at a globally important sea turtle nesting site. Glob. Change Biol. 2017;23:4922–4931. doi: 10.1111/gcb.13765. [DOI] [PubMed] [Google Scholar]
- 41.Wedekind Claus. Topics in Conservation Biology. 2012. Managing Population Sex Ratios in Conservation Practice: How and Why? [Google Scholar]
- 42.Girondot M, Fouillet H, Pieau C. Feminizing turtle embryos as a conservation tool. Conserv. Biol. 1998;12:353–362. doi: 10.1046/j.1523-1739.1998.96382.x. [DOI] [Google Scholar]
- 43.Houghton JDR, et al. Protracted rainfall decreases temperature within leatherback turtle (Dermochelys coriacea) clutches in Grenada, West Indies: ecological implications for a species displaying temperature dependent sex determination. Journal of experimental marine biology and ecology. 2007;345:71–77. doi: 10.1016/j.jembe.2007.02.001. [DOI] [Google Scholar]
- 44.Beger M, et al. Integrating regional conservation priorities for multiple objectives into national policy. Nat. Commun. 2015;6:8208. doi: 10.1038/ncomms9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cantarelli VH, Malvasio A, Verdade LM. Brazil’s Podocnemis expansa conservation program: retrospective and future directions. Chelonian Conserv. Biol. 2014;13:124–128. doi: 10.2744/CCB-0926.1. [DOI] [Google Scholar]
- 46.Esteban N, van Dam RP, Harrison E, Herrera A, Berkel J. Green and hawksbill turtles in the Lesser Antilles demonstrate behavioural plasticity in inter-nesting behaviour and post-nesting migration. Mar. Biol. 2015;162:1153–1163. doi: 10.1007/s00227-015-2656-2. [DOI] [Google Scholar]
- 47.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. www.R-project.org (2014).
- 48.Bates D, Maechler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]