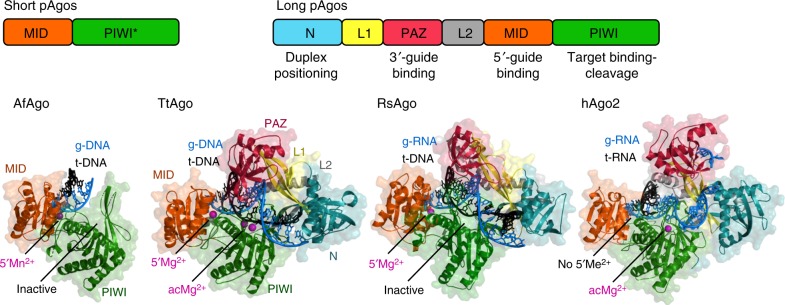
Fig. 1.
Structural organization of Ago proteins. The domain architecture of short and long pAgos is schematically illustrated at the top. Short pAgos always contain inactive PIWI domain (PIWI*). The structures of four representative Ago proteins are shown in ternary complexes with guide (“g-”) and target (“t-”) nucleic acids: short inactive AfAgo (PDB: 2W4217) and long active TtAgo with g-DNA and t-DNA (PDB: 4NCB30), long inactive RsAgo with g-RNA and t-DNA (PDB: 5AWH35) and active human Ago2 with g-RNA and t-RNA (PDB: 4W5O54). The N-domain is turquoise, L1 is yellow, PAZ is magenta, L2 is gray, MID is orange, PIWI is green. The guide strand is blue, the target strand is black. Metal ions bound in the MID-pocket (5′Me2+) or in the active center (acMe2+) are indicated