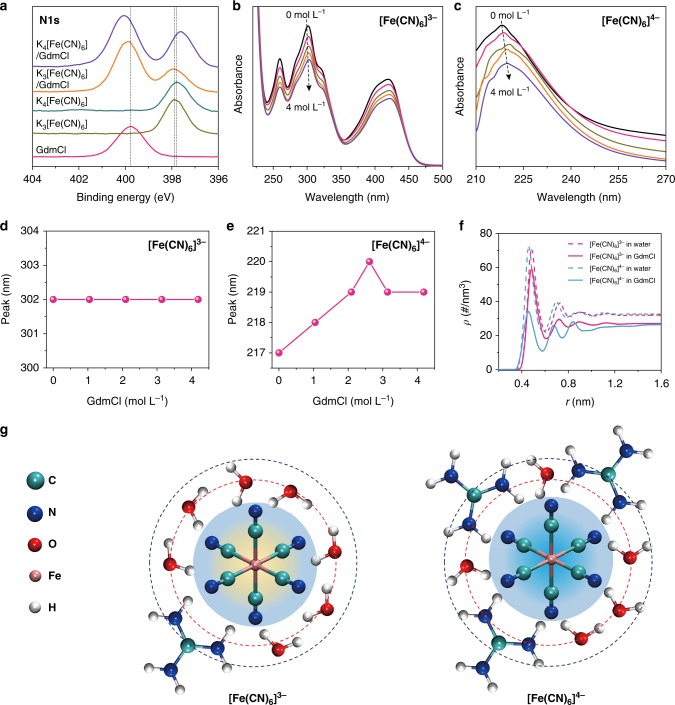
Fig. 2.
The mechanism of enhancement of the Seebeck effect by GdmCl. a XPS spectra shift for K3[Fe(CN)6], K4[Fe(CN)6], GdmCl, and their composites. For the composites, GdmCl at an optimized concentration of 2.6 M was added to 0.4 M K3[Fe(CN)6] or K4[Fe(CN)6]. The solid samples used for XPS measurement were prepared by drying the composite solutions in a vacuum oven at 333 K for 48 h. UV–Vis spectral shifts and the corresponding absorption peaks of [Fe(CN)6]3− (b, d) and [Fe(CN)6]4− (c, e) with increasing concentrations of GdmCl. f Radial density profiles of the hydrated [Fe(CN)6]3− and [Fe(CN)6]4− anions in pure water and GdmCl aqueous solution obtained from the results of MD simulation (Supplementary Note 1). g The schematic solvation formations of [Fe(CN)6]3− and [Fe(CN)6]4− in GdmCl solution