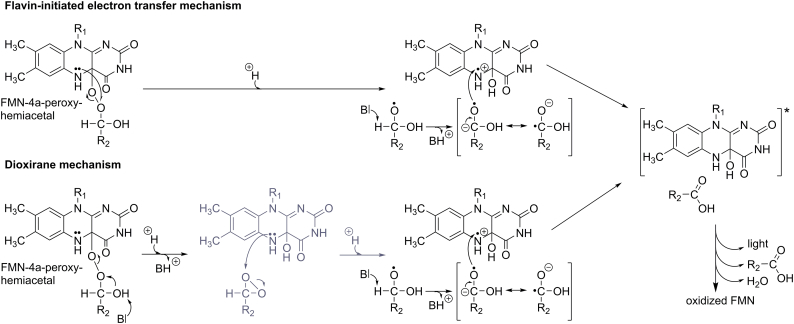
Scheme 5.
Mechanistic details of the flavin-initiated electron transfer and the dioxirane mechanism. In the former mechanism (upper reaction path), electron transfer from N5 of the isoalloxazine ring to the distal oxygen atom of the flavin-4a-peroxyhemiacetal (R1: ribityl monophosphate) leads to the formation of a substrate-derived alkoxy radical (e.g. R2: (CH2)12CH3) and the flavin-4a-hydroxy radical cation. Deprotonation of the alkoxy radical generates a resonance stabilized anion radical, which transfers an electron back to the flavin-4a-hydroxy radical cation thus leading to the population of the excited state of the flavin-4a-hydroxide. In an alternative route to this mechanism, the dioxirane mechanism (lower reaction path), the flavin-4a-peroxyhemiacetal forms a dioxirane intermediate, which then receives an electron from the flavin-4a-hydroxide (depicted in dark grey). As before, this leads to the flavin-4a-hydroxide radical cation and the subsequent generation of the excited state similar to the mechanism in the flavin-initiated electron transfer mechanism. In both reactions the rate limiting step is the electron donation from the reduced flavin moiety to the substrate moiety.