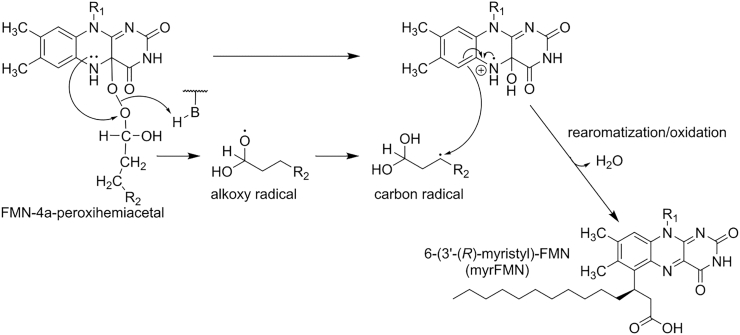
Scheme 6.
Proposed mechanism for myrFMN formation (adapted from Scheme 3 in Tabib, Brodl, Macheroux, Mol. Microbiol. 2017 [29]). As shown in Scheme 5, an electron is transferred from the N5 of the flavin to the distal oxygen atom of the peroxy moiety. A hydrogen rearrangement of the alkoxy radical (R2: (CH2)10CH3) intermediate leads to a C3 carbon radical. This combines with the flavin-4a-hydroxide radical cation forming a covalent bond between the C6 of the isoalloxazine ring and the C3 carbon of the myristyl aldehyde. After rearomatization and the oxidation of the aldehyde to the acid followed by release of water, 6-(3′-(R)-myristyl)-FMN (myrFMN) is formed.