Abstract
A 68-year-old man was admitted to our hospital for the further examination of intermittent claudication. He had been on continuous ambulatory peritoneal dialysis for 2 years. Screening transthoracic echocardiography (TTE) revealed a club-shaped tumor and a round-shaped tumor attached to mitral annulus calcification (MAC). The club-shaped tumor was swinging and plunged into the left ventricle at diastolic phase. Because of the risk of fatal embolism, we planned early surgical resection of the tumors. However, 13 days after admission, his intermittent claudication was getting worse and some part of the club-shaped tumor had vanished by TTE. Urgent iliac angiography showed that the tumor had embolized the right common iliac artery. Although we tried embolectomy using a Fogarty catheter, it was unsuccessful. We therefore treated the iliac artery stenosis by endovascular therapy and the procedure was successful. Three months later, he suffered from unstable angina and was treated by percutaneous coronary intervention. However, subacute stent thrombosis occurred after one month. After urgent treatment, we decided to treat him by coronary artery bypass graft and surgical resection of the residual tumor on MAC. The operation was performed successfully. Finally, the tumor was diagnosed as cardiac calcified amorphous tumor by its histologic features.
<Learning objective: Cardiac calcified amorphous tumor (CAT) is a rare, non-neoplastic cardiac tumor. Mobile and pedunculated cardiac CAT is considered to be an important risk of systemic embolism. Based on our case and previous reports we reviewed cardiac CAT, especially MAC-related CAT, and it appears to be related to end-stage renal disease and may grow within a short duration. It is important to perform routine serial echocardiography for hemodialyzed patients in whom MAC has been identified.>
Keywords: Cardiac CAT, MAC-related CAT, Acute embolism, Thrombosis, End-stage renal disease
Introduction
Cardiac calcified amorphous tumor (CAT) is a rare, non-neoplastic cardiac tumor. It was originally described by Raynolds et al. [1], and only 38 cases have so far been reported in the English literature. Cardiac CAT was also reported as a risk of embolism. In 9 of the 38 cases, embolic events suspected to be due to cardiac CAT were described. We recently encountered a case of cardiac CAT that caused right common iliac artery embolism and was successfully treated by endovascular therapy and surgical resection.
Case report
A 68-year-old man was admitted to our hospital for the further examination of intermittent claudication. He had been on continuous ambulatory peritoneal dialysis due to diabetic nephropathy for 2 years and had undergone percutaneous coronary intervention for recent myocardial infarction.
In physical examination, Levine II/VI ejection systolic murmur was audible at the apex, and pulsation of bilateral femoral arteries was good, although pulsation of the popliteal artery was weak. In a physiological test, ankle-brachial index (ABI) was low (right, 0.78; left, 0.85), and complete right bundle branch block was found by electrocardiography. In laboratory data, serum levels of creatinine, urea nitrogen, calcium, and phosphate were 13.6 mg/dl, 52 mg/dl, 8.4 mg/dl, and 6.5 mg/dl, respectively. And parathyroid hormone level was within the normal range.
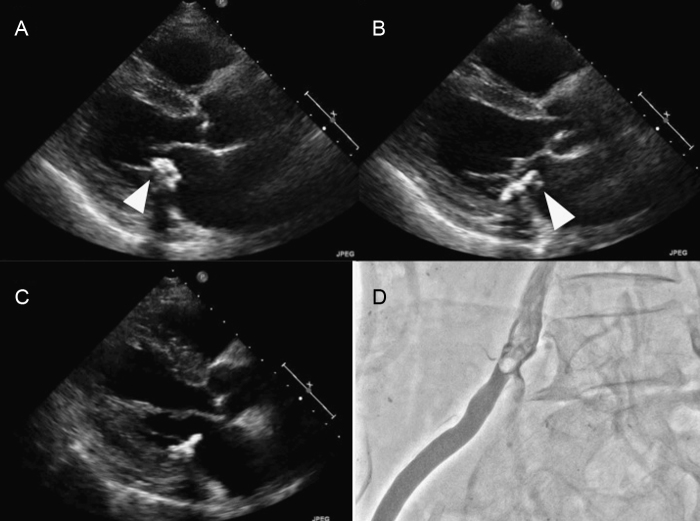
Transthoracic echocardiography (TTE) for cardiac screening revealed two intracavitary calcified tumors attached to a large mitral annulus calcification (MAC) (Fig. 1A and B). A club-shaped tumor (13 mm × 14 mm) was attached to the left atrium (lateral side of the MAC), and a round-shaped tumor (8 mm × 8 mm) was present in the left ventricle (medial side of the MAC). The club-shaped tumor attached to the MAC had high mobility and was swinging and it plunged into the left ventricle at diastolic phase, although the round-shaped tumor did not have any mobility. We speculated that the tumors had developed in a short duration because routine TTE 10 months before hospitalization revealed no detectable tumors, although a large size of MAC was already observed.
Fig. 1.
(A) Transthoracic echocardiography (parasternal long-axial view) before embolism (diastolic phase): a club-shaped tumor (13 mm × 14 mm) plunged into left ventricle at diastolic phase (white arrow). (B) Transthoracic echocardiography before embolism (systolic phase). (C) Transthoracic echocardiography after embolism (diastolic phase): apical portion of the club-shaped tumor had vanished. (D) Iliac artery angiography: radiolucent lesion at the bifurcation of internal and external iliac arteries.
We planned surgical resection of the tumors because of the risk of fatal embolism. In the preoperative examination, coronary artery angiography revealed triple vessel disease, and we therefore planned surgical resection of the tumors and coronary artery bypass graft.
However, 13 days after admission, his claudication had worsened and echocardiography revealed that the apical portion of the club-shaped tumor had vanished, although the pedicle of the tumor remained on the MAC (Fig. 1C). On physical examination, pulsation of the right femoral artery was obviously weak. ABI of the right foot had worsened from 0.78 to 0.55. Other physical examination and imaging tests did not show the embolism in other organs.
On urgent iliac artery angiography, we found a radiolucent lesion at the bifurcation of the internal and external iliac arteries (Fig. 1D). We tried urgent embolectomy by using a Fogarty catheter, but it was unsuccessful because of balloon rupture. We then tried endovascular therapy to improve blood flow in the external iliac artery. With a cross-over approach from the left femoral artery, we successfully dilated the iliac artery by nitinol stent implantation. After this therapy, the patient's intermittent claudication improved and ABI of his right foot also improved from 0.55 to 0.69. Although the pedicle of the tumor remained on the MAC, we did not try to surgically resect the tumors because of the high risk of postoperative cerebral infarction (due to severe stenosis of the middle cerebral artery).
Three months after iliac embolism, he suffered from unstable angina of the right coronary artery. When the coronary artery event occurred, the pedicle of the tumor remaining on the MAC did not change apparently by echocardiography. We tried to treat him by percutaneous coronary intervention, and the intervention with rotational atherectomy and using drug-eluting stents was successfully performed. However, as subacute stent thrombosis occurred one month after percutaneous coronary intervention, we therefore decided to treat him by coronary artery bypass graft despite the risk of cerebral infarction, and we tried surgical resection of the remaining pedicle of the cardiac tumor.
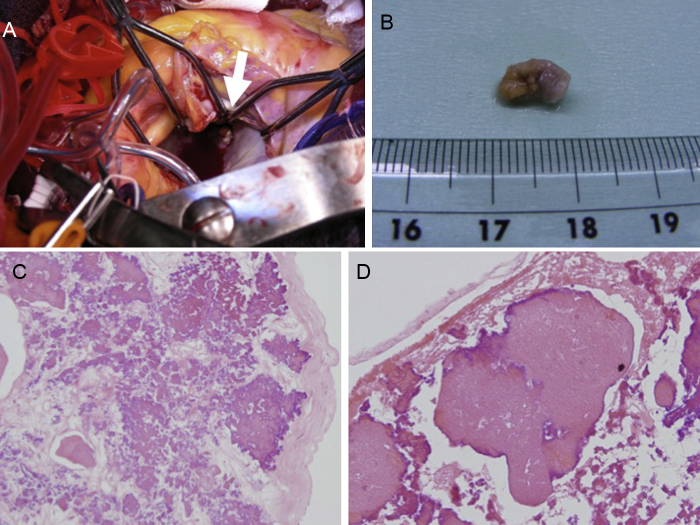
In the operation, we found the residual pedicle of the tumor on the atrial side of the MAC (10 mm × 15 mm) and surgically resected it (Fig. 2A and B). The round-shaped tumor on the ventricular side was not identified in the operation because of the large MAC. The patient recovered uneventfully and was discharged.
Fig. 2.
(A) Intraoperative findings: yellowish-white tumor was detected on the left atrial side of mitral annulus calcification. (B) Photograph of the excised tumor. Round, yellowish-white tumor (10 mm × 8 mm) was resected in the operation. (C, D) Pathological findings. (C) Large and nodal calcium deposits within a background of eosinophilic, amorphous material (hematoxylin and eosin; 20×). (D) Small amount of fresh fibrin was detected in the peripheral zone of the tumor (hematoxylin and eosin; 40×).
In pathological examination, the cardiac tumor consisted of large and nodal calcium deposits within a background of eosinophilic, amorphous material and fresh fibrin, and multinucleated giant cells were also detected around the calcium deposits (Fig. 2C and D).
We finally diagnosed the tumor as cardiac CAT because of its echocardiographic and pathological characteristics.
Discussion
Cardiac CAT is a rare, non-neoplastic cardiac tumor. It was originally described in 1997 by Reynolds et al. [1]. They retrospectively evaluated 11 cases of intracavitary non-neoplastic cardiac tumors and found the same clinical and pathological characteristics. They defined this group of tumors as cardiac CAT based on their pathological findings. Since the report by Reynolds et al., reports of 38 cases of cardiac CAT have been published in the English literature, that were searched for by Pubmed (only 2 of them being English abstracts). In those cases, cardiac CAT developed in all chambers of the heart, many of them had a pedicle, and they mimicked cardiac neoplasm, vegetation or thrombus. Their pathological characteristics were nodular deposits of calcium with a background of eosinophilic amorphous materials and fresh fibrin. These clinical and pathological characteristics are identical to those of our case.
In addition, 10 of the 39 cases of cardiac CAT including our case were associated with MAC 2, 3, 4, 5, 6, 7. MAC-related CAT cases were reported to have some uncommon clinical and echocardiographic features (Table 1). They were strongly related to end-stage renal disease and hemodialysis (8 of the 10 cases having end-stage renal disease), had a high rate of pedunculated tumors (8/10), had high mobility in echocardiography, and mimicked vegetation in clinical diagnosis. Because of these features, MAC-related CAT is now suspected to be an atypical variant of cardiac CAT. Although Reynolds et al. considered a cardiac CAT as organized thrombus because of its pathological features and clinical situation (some of their cases had a factor of hypercoagulability) [1], the etiology of cardiac CAT is still unclear. In addition, MAC-related CAT may have different etiology from that of cardiac CAT. Tsuchihashi et al. reported 4 cases of “mobile intracardiac calcinosis” in patients with end-stage renal disease in 1999 [8]. They focused on secondary hyperparathyroidism of hemodialyzed patients as etiology of these tumors, and they speculated that imbalance in serum calcium and phosphate might play an important role in the development of these tumors. If the intracardiac calcinosis and cardiac CAT are based on the same etiology, organized thrombosis may not be the only mechanism for cardiac CAT; abnormal calcium metabolism and chronic inflammation in hemodialyzed patients may also play an important role in the development of cardiac CAT, especially MAC-related CAT.
Table 1.
Clinical findings in 10 cases of calcified amorphous tumor-related mitral annulus calcification.
| Reference | Age/sex | Symptom | Pedicle | ESRD | Embolism | Tumor size (cm) | Tumor site | Therapy | Underlying disease |
|---|---|---|---|---|---|---|---|---|---|
| Morishima et al. [2] | 68/M | Dyspnea | + | Dialysis | – | 0.7 × 0.8 | LA, MV (PML) | Surgery | ESRD, DM, HT, CAD |
| 63/M | Dyspnea chest compression | − | CAPD | – | 2 × 1.5 × 1.7 | LA, MV (PML) | Surgery | ESRD, CHF | |
| Kubota [3] | 64/F | Asymptomatic | + | Dialysis | – | 0.3 × 2.7 | LV, MV (AML) | Surgery | DM nephropathy |
| 44/M | Asymptomatic | + | Dialysis | – | 0.5 × 2.8 | LV | Surgery | Lupus nephritis | |
| Fujiwara et al. [4] | 58/M | Asymptomatic | + | Dialysis | – | Not described | LA + LV | Surgery | DM nephropathy |
| 65/M | Asymptomatic | + | Dialysis | – | Not described | LV | Surgery | Chronic glomerulonephritis | |
| Nishigawa et al. [5] | 78/F | Asymptomatic | + | – | – | 0.17 | LA | Surgery | – |
| Kawata et al. [6] | 59/M | Asymptomatic | + | Dialysis | – | 0.6 × 2.8 | LV | Surgery | DM nephropathy |
| Yamamoto et al. [7] | 82/F | Heart failure | + | – | – | 0.4 × 3.7 | LV, MV (PML) | Surgery | – |
| Current case | 68/M | Intermitted claudication | + | CAPD | Iliac artery embolism | 1.3 × 1.4 | LA + LV | Surgery | DM nephropathy, HT, CAD |
ESRD, end-stage renal disease; CAPD, continuous ambulatory peritoneal dialysis; MAC, mitral annulus calcification; LA, left atrium; LV, left ventricle; PML, posterior mitral leaflet; AML, anterior mitral leaflet; DM, diabetes mellitus; HT, hypertension; CAD, coronary artery disease; CHF, congestive heart failure.
Willens et al. reported two cases of cardiac mobile mass in 2003 [9]. They reported that a highly echoic, mobile mass rapidly developed on the MAC and was totally resolved by antiplatelet therapy without embolism. They also reviewed 18 cases with mobile components associated with MAC and revealed that some of the masses were resolved with anticoagulation or antiplatelet therapy. According to their report, organized thrombosis associated with MAC may be the main etiology of MAC-related CAT, and abnormal calcium metabolism may transform those thrombi into more calcified, highly echoic tumors.
In our case, the cardiac CAT on the MAC was thought to have formed within 10 months because routine echocardiography had revealed no detectable tumors 10 months before hospitalization. Although the exact cause of cardiac CAT is still unknown, the hypothesis of organized thrombi may be able to explain such rapid growth of a cardiac CAT.
Including our case, 10 of the 39 cases of cardiac CAT had some embolic events, including cerebral infarction, pulmonary embolism, and retinal artery embolism [10]. Cardiac CAT, especially mobile and pedunculated cardiac CAT, is considered to be an important risk for systemic embolism. However, to our knowledge, embolic events associated with MAC-related CAT are relatively rare and this is the first case of embolism due to MAC-related CAT.
Based on our case and previous reports [6], cardiac CAT, especially MAC-related CAT, may grow within a short duration. To prevent systemic embolism, routine serial echocardiography is recommended for hemodialyzed patients in whom MAC has been identified.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Reynolds C., Tazelaar H.D., Edwards W.D. Calcified amorphous tumor of the heart (cardiac CAT) Hum Pathol. 1997;28:601–606. doi: 10.1016/s0046-8177(97)90083-6. [DOI] [PubMed] [Google Scholar]
- 2.Morishima A., Sasahashi N., Ueyama K. Calcified amorphous tumors with excision in hemodialysis patient: report of 2 cases. Kyobu Geka. 2006;59:851–854. [PubMed] [Google Scholar]
- 3.Kubota H., Fujioka Y., Yoshino H., Koji H., Yoshihara K., Tonari K., Endo H., Tsuchiya H., Mera H., Soga Y., Taniai S., Sakata K., Sudo K. Cardiac swinging calcified amorphous tumors in end-stage renal failure patients. Ann Throrac Surg. 2010;90:1692–1694. doi: 10.1016/j.athoracsur.2010.04.097. [DOI] [PubMed] [Google Scholar]
- 4.Fujiwara M., Watanabe H., Iino T., Kobukai Y., Ishibashi K., Yamamoto H., Iino K., Yamamoto F., Ito H. Two cases of cardiac calcified amorphous tumor mimicking mitral valve vegetation. Circulation. 2012;125:e432–e434. doi: 10.1161/CIRCULATIONAHA.111.072793. [DOI] [PubMed] [Google Scholar]
- 5.Nishigawa K., Takiuchi H., Kubo Y., Masaki H., Yanemoto K. Calcified amorphous tumor: three dimensional transesophageal echocardiography. Asian Cardiovasc Thorac Ann. 2012;20:355. doi: 10.1177/0218492311423071. [DOI] [PubMed] [Google Scholar]
- 6.Kawata T., Konishi H., Amano A., Daida H. Wavering calcified amorphous tumour of the heart in a haemodialysis patient. Interact Cardiovasc Thorac Surg. 2013;16:219–220. doi: 10.1093/icvts/ivs430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto M., Nishimori H., Wariishi S., Fukutomi T., Kondo N., Kihara K., Tashiro M., Tanioka K., Orihashi K. A cardiac calcified amorphous tumor stuck in the aortic valve that mimicked a chameleon's tongue: report of a case. Surg Today. 2014;44:1751–1753. doi: 10.1007/s00595-013-0698-y. [DOI] [PubMed] [Google Scholar]
- 8.Tsuchihashi K., Nozawa A., Marusaki S., Moniwa N., Oh-numa Y., Kuno A., Takagi S., Takizawa H., Ura N., Shimamoto K. Mobile intracardiac calcinosis: a new risk of thromboembolism in patients with haemodialysed end stage renal disease. Heart. 1999;82:638–640. doi: 10.1136/hrt.82.5.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willens H.J., Ferreira A.C., Gallagher A.J., Morytko J.A. Mobile components associated with rapidly developing mitral annulus calcification in patient with chronic renal failure: review of mobile elements associated with mitral annulus calcification. Echocardiography. 2003;20:363–367. doi: 10.1046/j.1540-8175.2003.03042.x. [DOI] [PubMed] [Google Scholar]
- 10.Nazli Y., Colak N., Atar I.A., Alpay M.F., Haltas H., Eryonucu B., Cakir O. Sudden unilateral vision loss arising from calcified amorphous tumor of the left ventricle. Tex Heart Inst J. 2013;40:453–458. [PMC free article] [PubMed] [Google Scholar]