Table 5.
VBeta T cell signatures and Streptococcus pyogenes toxin gene profiles of patients with streptococcal toxic shock syndrome.
| Treatments | Immunological data | Microbiological data | ||||||
|---|---|---|---|---|---|---|---|---|
| Gender | Antitoxin antibiotic (Clindamycin) | IVIGa (Dose) | Vβ alterations of CD3+ T cells (%)b | Vβ alterations of CD3+ T cells (%) | Toxin suspected according to Vβ modification profile | Site(s) of isolation | emm-types | Toxin gene profile |
| Measurement 1 [day post shock onset] | Measurement 2 [day post shock onset] | |||||||
| Fc | Yes | Yes (2 g/kg) | Vβ1 ↔ (4.6%); Vβ2 ↔ (5.8%); Vβ5.1 ↔ (5.6%); Vβ12 ↔ (1.5%); Vβ14  (5.6%) [Dd+3] (5.6%) [Dd+3] |
Vβ1  (5.6%); Vβ2 ↔ (9.22%); Vβ5.1 ↔ (4.24%); Vβ12 ↔ (1.32%); Vβ14 (5.6%); Vβ2 ↔ (9.22%); Vβ5.1 ↔ (4.24%); Vβ12 ↔ (1.32%); Vβ14  (5.1%) [Dd+8] (5.1%) [Dd+8] |
SPEAe or SPECf | Throat | 89 | speBg, speCh |
| Mi | Yes | No | Vβ1 ↔ (2.2%); Vβ2 ↔ (10.6%); Vβ5.1 ↔ (4.7%); Vβ12  (5.8%); Vβ14 (5.8%); Vβ14  (22.1%) [D+4] (22.1%) [D+4] |
NDj | SPEA | Pleural effusion | 1 | speAk speB |
| F | Yes | Yes (2 g/kg) | Vβ1  (8.2%); Vβ2 (8.2%); Vβ2  (11.8%); Vβ5.1 ↔ (3.4%); Vβ12 ↔ (1.9%); Vβ14 ↔ (4.4%) [D+3] (11.8%); Vβ5.1 ↔ (3.4%); Vβ12 ↔ (1.9%); Vβ14 ↔ (4.4%) [D+3] |
ND | SPEC | Blood | 87 | speB, speC |
| M | Yes | Yes (2 g/kg) | Vβ1 ↔ (2.3%); Vβ2  (19.1%); Vβ5.1 (19.1%); Vβ5.1 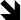 (3.3%); Vβ12 (3.3%); Vβ12  (2.3%); Vβ14 (2.3%); Vβ14  (15.8%) [D+3] (15.8%) [D+3] |
ND | SPEA | Pleural effusion, blood | 1 | speA, speB |
| M | Yes | Yes (1 g/kg) | Vβ1 ↔ (2.9%); Vβ2  (27.6%); Vβ5.1 (27.6%); Vβ5.1 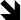 (2.5%); Vβ12 ↔ (1.4%); Vβ14 (2.5%); Vβ12 ↔ (1.4%); Vβ14  (6.8%) [D+1] (6.8%) [D+1] |
ND | SPEA or SPECj | Pleural effusion, blood | 12 | speB, speC |
| F | Yes | Yes (NAl) | Vβ1 ↔ (2.5%); Vβ2  (15.4%); Vβ5.1 (15.4%); Vβ5.1 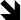 (3.4%); Vβ12 (3.4%); Vβ12  (3.6%); Vβ14 (3.6%); Vβ14  (17.7%) [D+4] (17.7%) [D+4] |
ND | SPEA | Pleural effusion | 1 | speA, speB |
| F | Yes | Yes (1 g/kg) | ND | ND | ND | Wound | 28 | speB, speC |
| M | Yes | Yes (2 g/kg) | ND | ND | ND | Throat | ND | ND |
| F | No | No | ND | ND | ND | Blood | ND | ND |
| F | No | No | ND | ND | ND | Pleural effusion | 1 | speA, speB, speC |
| M | Yes | No | ND | ND | ND | Blood | 12 | speB |
| F | Yes | No | ND | ND | ND | Pleural effusion, blood | 1 | speA, speB |
| M | Yes | No | ND | ND | ND | Tracheal secretions | ND | ND |
| F | Yes | No | ND | ND | ND | Pleural effusion, tracheal secretions | 6 | speB, speC |
| M | Yes | Yes (2 g/kg) | ND | NDl | ND | Pleural effusion | 1 | speA, speB, speC |
IVIG, Intravenous Immunoglobulin.
Vβ, alterations of CD3+ T cells (%): The expression of 24 main Vβ CD3+ T cells was determined by flow cytometry. Only the altered expression of Vβ CD3+ T cells is showed according the following normal adult ranges provided by the kit manufacturer: Vβ1: 2.18 to 4.88%Vβ2: 5.84 to 10.76%; Vβ5.1: 3.85 to 7.05%; Vβ12: 1.12 to 2.2%; Vβ14: 2.13 to 4.85%.
F, Female.
D, day.
SPEA, streptococcal pyrogenic exotoxin A.
SPEC, streptococcal pyrogenic exotoxin C.
speB, gene encoding streptococcal pyrogenic exotoxin B.
speC, gene encoding streptococcal pyrogenic exotoxin C.
M, male.
ND, not determined.
speA, gene encoding streptococcal pyrogenic exotoxin A.
NA, not available.
No specific other fundings for this study.
