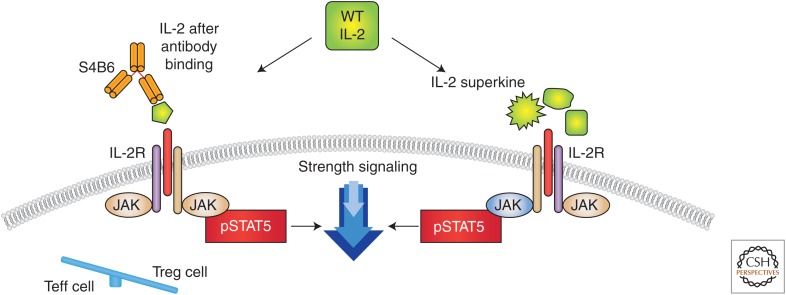
Figure 3.
Different approaches to change interleukin (IL)-2 conformation to favor effector over negative regulatory function. One strategy for selectively modulating the effects of IL-2 is the development of cytokine-directed antibodies that bias activity toward specific T-cell subsets. The anti-IL-2 antibody S4B6 blocked the IL-2:IL-2Rα interaction while also conformationally stabilizing the IL-2:IL-2Rβ interaction, thus stimulating all IL-2-responsive immune cells, particularly IL-2Rβhi effector cells favored over IL-2Rα expressing regulatory T (Treg) cells. The right side shows a different mutational approach to generate a distinct IL-2R superkine variance, which uses mutations to stabilize IL-2, including a flexible helix in the IL-2Rβ-binding site, into an optimized receptor-binding confirmation resembling that when bound to CD25 (IL-2Rα) (Levin et al. 2012). The evolved mutations in super-2 recapitulated the functional role of CD25 by eliciting potential phosphorylation of signal transducers and activators of transcription 5 (STAT5) and vigorous proliferation of T cells irrespective of CD25 expression. Compared to IL-2, super-IL-2 induced superior expansion of cytotoxic T cells, leading to improved antitumor responses in vivo and eliciting proportionally less expansion of Treg cells and reduced pulmonary edema. WT, Wild type.