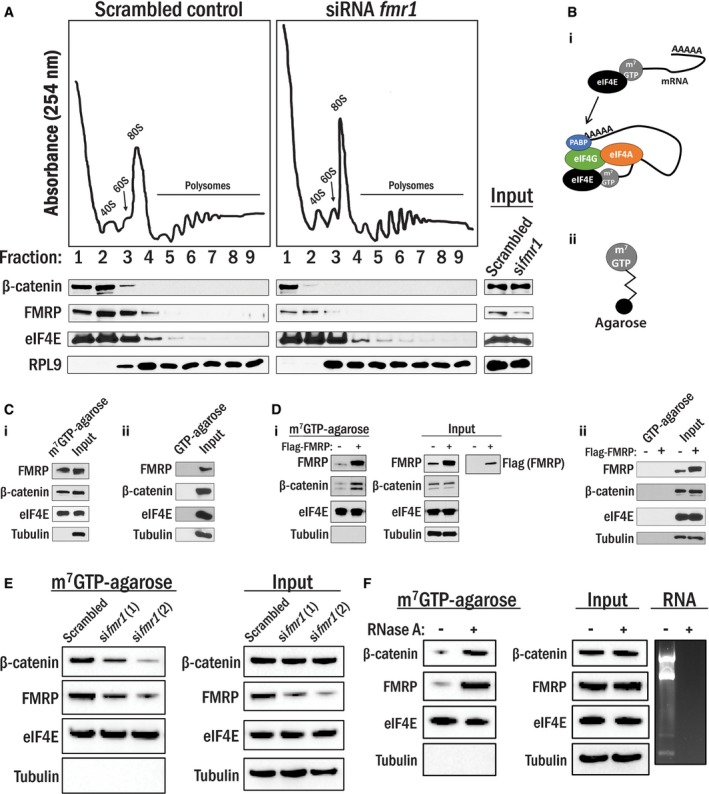
Co‐sedimentation of protein/RNA complexes on a 10‐50% sucrose gradient. Cell lysates generated from the cells transfected with either siRNA targeting fmr1 or its scrambled control were ultracentrifuged in sucrose gradients; peaks corresponding to the 40S and 60S subunits, 80S monosome, and polysomes were detected by UV absorbance at 254 nm, and indicated proteins in these fractions were detected by Western blot. Corresponding total cell lysate was used as input.
(i) eIF4E preferentially binds to the 5′cap (m7GTP) of mRNAs and recruits the pre‐initiation complex to that site. (ii) m7GTP‐agarose beads.
(i) Precipitated proteins in A10 cell lysates with m7GTP‐agarose beads were identified by Western blot analysis. eIF4E and tubulin were shown as positive and negative controls, respectively. Total lysates were used as input. (ii) A10 cell lysates were incubated with GTP‐agarose beads, and none of the proteins tested were precipitated with the beads. Total lysates were used as input control.
(i) HEK 293T cells were transfected with empty vector or Flag‐FMRP, and lysates were subjected to m7GTP‐agarose pull‐down as in (C). (ii) HEK 293T cells were transfected with empty vector or Flag‐FMRP, and lysates were subjected to GTP‐agarose pull‐down as in (i).
HEK 293T cells were transfected with either siRNAs targeting fmr1 or scrambled control and were subjected to m7GTP‐agarose pull‐downs as in (C).
HEK 293T cells lysates were subjected to m7GTP‐agarose pull‐downs as in (C) in the presence or absence of RNase A (10 μg/ml). RNA was extracted from parallel lysates, and RNA content was analyzed by agarose gel electrophoresis.