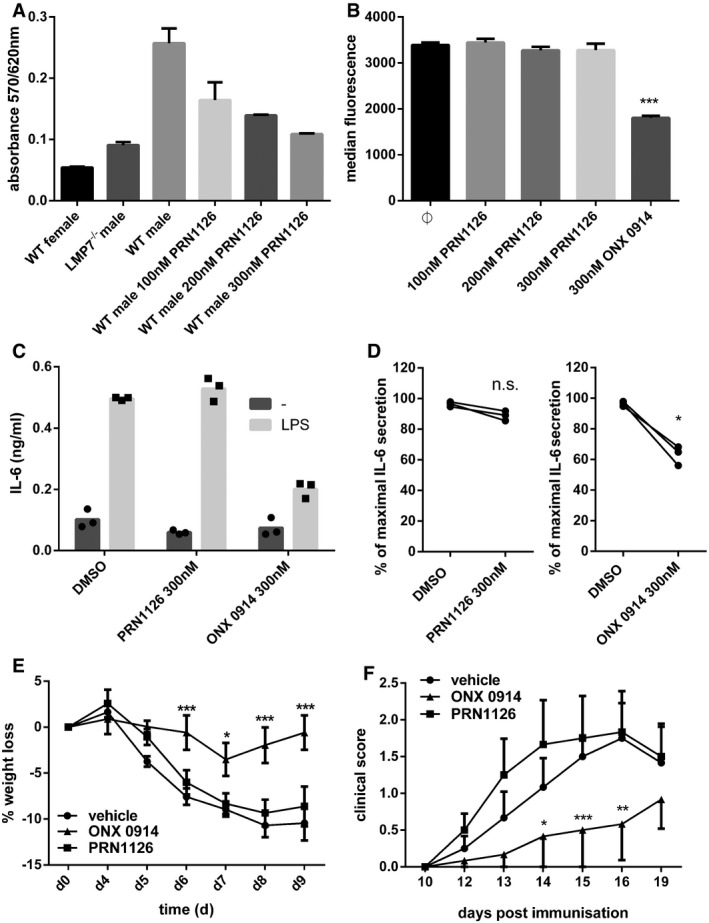
-
A
Presentation of UTY246–254 on splenocytes after exposure to indicated concentrations of PRN1126. Data are presented as the mean absorbance ± s.d. of three replicate cultures. The experiment has been performed twice, yielding similar results.
-
B
Flow cytometry analysis of H‐2Kb surface expression on splenocytes derived from C57BL/6 mice treated with the indicated concentrations of PRN1126 overnight. Pooled data from three independent experiments (n = 9) are shown as the means of median fluorescence intensity ± s.e.m. All data were statistically compared to the DMSO‐treated group. ***P < 0.001. One‐way ANOVA.
-
C, D
Splenocytes from C57BL/6 mice (C) or human PBMCs (D) were exposed (continuous treatment) to 300 nM PRN1126, or vehicle (DMSO), or 300 nM ONX 0914 and stimulated with LPS overnight. IL‐6 concentrations in the supernatant were analyzed by ELISA. (C) IL‐6 concentrations are presented as mean and individual data points from triplicate wells. The experiment has been performed twice, yielding similar results. (D) Data are presented as single dots from three independent donors. The highest cytokine concentration was set to 100%. *P < 0.05. Unpaired Student's t‐test.
-
E
Colitis was induced by oral administration of 3% DSS for 5 days. Mice were treated daily (s.c.) with either PRN1126 (40 mg/kg), or ONX 0914 (10 mg/kg), or vehicle. Data points represent mean ± s.e.m. of 15 mice pooled from three independent experiments. All data were statistically compared to the vehicle‐treated group. *P < 0.05, ***P < 0.001. Two‐way ANOVA.
-
F
Mice were immunized with MOG35–55 peptide and were monitored daily for clinical symptoms of EAE. Mice were treated three times a week (s.c.) with either PRN1126 (40 mg/kg), or ONX 0914 (10 mg/kg), or vehicle. All data were statistically compared to the vehicle‐treated group. *P < 0.05, **P < 0.01, ***P < 0.001. Two‐way ANOVA. Shown are the means of the clinical scores ± s.e.m. (n = 6 per group). The experiments have been performed twice, yielding similar results.