Abstract
Background
The inconsistent ability of novel biomarkers to predict acute kidney injury (AKI) across heterogeneous patients and illnesses limits integration into routine practice. We previously retrospectively validated the ability of the renal angina index (RAI) to risk-stratify patients and provide context for confirmatory serum biomarker testing for the prediction of severe AKI.
Methods
We conducted this first prospective study of renal angina to determine whether the RAI on the day of admission (Day0) risk-stratified critically ill children for ‘persistent, severe AKI’ on Day 3 (Day3-AKI: KDIGO Stage 2–3) and whether incorporation of urinary biomarkers in the RAI model optimized AKI prediction.
Results
A total of 184 consecutive patients (52.7% male) were included. Day0 renal angina was present (RAI ≥8) in 60 (32.6%) patients and was associated with longer duration of mechanical ventilation (P = 0.04), higher number of organ failure days (P = 0.003) and increased mortality (P < 0.001) than in patients with absence of renal angina. Day3-AKI was present in 15/156 (9.6%) patients; 12/15 (80%) fulfilled Day0 renal angina. Incorporation of urinary biomarkers into the RAI model increased the specificity and positive likelihood, and demonstrated net reclassification improvement (P < 0.001) for the prediction of Day3-AKI. Inclusion of urinary neutrophil gelatinase-associated lipocalin increased the area under the curve receiver-operating characteristic of RAI for Day3-AKI from 0.80 [95% confidence interval (CI): 0.58, 1.00] to 0.97 (95% CI: 0.93, 1.00).
Conclusions
We have now prospectively validated the RAI as a functional risk stratification methodology in a heterogeneous group of critically ill patients, providing context to direct measurement of novel urinary biomarkers and improving the prediction of severe persistent AKI.
Keywords: acute kidney injury, biomarkers, critical care, risk stratification, renal angina index
INTRODUCTION
Acute kidney injury (AKI) occurs frequently in critically ill patients and is independently associated with high rates of morbidity and mortality. In a recent meta-analysis of over 3.5 million patients, the incidence of AKI was 21.6% (one in five) in adults and 33.7% in children (one in three), with mortality rates of 23.9 and 13.8%, respectively [1]. Since the adoption of severity-based classifications of injury [2–6], there has been an increasing awareness of the danger of AKI and the associated negative clinical impact of a kidney ‘attack’ [7, 8]. Unfortunately, no singular effective therapy for AKI has been developed and management consists largely of supportive care [9]. The Kidney Diseases: Improving Global Outcomes (KDIGO) working group recently described a stage-based best practice AKI management algorithm, notably highlighting recommendations after recognition of a patient with subclinical AKI or AKI risk [4].
Prediction of AKI or risk-stratifying patients in danger of kidney damage offers the opportunity to initiate measures for AKI prevention. Creatinine-based detection strategies, limited in efficacy by time-lag in response to injury and variability with body mass and gender, are particularly problematic in the pediatric population [10]. Novel biomarkers have demonstrated superior performance for the prediction of AKI compared with early, non-stratified changes in serum creatinine (SCr). Validated following cardiopulmonary bypass (CPB) in children, urinary neutrophil gelatinase-associated lipocalin (uNGAL), kidney injury molecule-1 (uKIM-1), interleukin 18 (uIL-18), liver type-fatty acid binding protein (uL-FABP) and cell cycle markers insulin-like growth factor binding protein 7 (IGFBP7) and tissue inhibitor of metalloproteinases-2 (TIMP-2) detect AKI prior to the functional change heralded by an SCr rise [11–14]. The pediatric CPB population, however, represents an isolated and unique cohort in which to validate AKI biomarkers, as most patients are without other acute illness, have a known time and duration of insult, and are rigorously monitored during and following injury. Unfortunately, a decade after the seminal report describing the exceptional discrimination of urine and serum NGAL for AKI following CPB [15], and despite growing support from the critical care nephrology community [16], AKI biomarkers have yet to be incorporated into routine practice. The reticence to acceptance of the biomarkers may reflect the inconsistent performance of these biomarkers for the prediction of AKI outside of the cardiac surgery population [17]. In children and adults, a majority of area under the curve receiver-operating characteristic (AUC-ROC) values of novel biomarkers for the prediction of AKI in the non-cardiac surgery population are between 0.6 and 0.8 [17]. Newer biomarkers reflecting injury to different parts of the nephron and injury of different types (i.e. cell cycle arrest) may have potential for utility in the broader population of critically ill patients, and demonstrate superior performance (AUC ∼0.9) [18]. Still, even the most widely studied novel biomarker, NGAL, demonstrates inconsistent performance depending on the population tested [e.g. adults in the emergency department, children with sepsis, adult intensive care unit (ICU) patients] [19–22].
Context-driven testing enhances biomarker utility. Cardiac–troponin I (cTi) has a high false positive rate for acute coronary syndrome (ACS) when tested in patients without chest pain and risk factors for heart disease [23]. Akin to heart attack, kidney attack carries a prodrome, providing context for novel biomarker testing [8, 24]. We previously derived and validated the renal angina model to stratify patients by risk of persistent, severe AKI [25]. Modeled on the empiric concept of organ ‘torment, suffering, injury’ (from the etymology of ankhone or angina), renal angina describes a state of kidney injury identifiable using the renal angina index (RAI). The RAI is an easily calculable measure of renal angina combining objective signs of kidney dysfunction and patient context (i.e. risk factors for AKI) (Figure 1). In a convenience sample population studied retrospectively, the RAI demonstrated an above-average AUC-ROC for the prediction of severe and persistent AKI (0.80), improving significantly with the incorporation of a positive serum AKI biomarker (0.84–0.88) [26]. We now present data from the first prospective study of renal angina testing the hypothesis that incorporation of urinary biomarkers in patients who ‘rule in’ for renal angina (RAI ≥8) results in robust risk stratification for the development of severe, persistent AKI.
FIGURE 1:
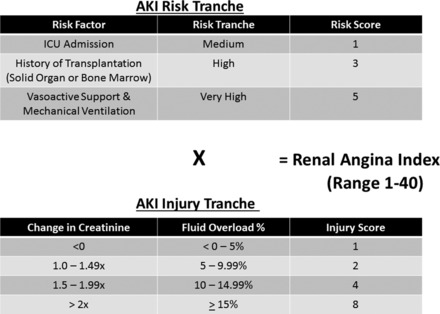
The RAI: based on existing pediatric AKI literature, tiered AKI risk strata were assigned point values for ‘risk’ and ‘signs’ of injury. The worse parameter between change in estimated creatinine clearance from baseline and %FO was used to yield an injury score. The resultant RAI score can range from 1 to 40. A cutoff of >8 is used to determine renal angina fulfillment (from Basu et al. [25] with permission). ppCRRT, prospective pediatric continuous renal replacement therapy registry.
MATERIALS AND METHODS
Patients
This investigation was approved by the Institutional Review Board at Cincinnati Children's Hospital Medical Center (CCHMC) with waiver of the need for informed consent. The Acute Kidney Injury in CHildren Expected by Renal angina and Urinary Biomarkers (AKI-CHERUB, NCT01735162) was a prospective observational study conducted in the CCHMC pediatric ICU (PICU) from September 2012 to March 2014. Study procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
We prospectively enrolled all children admitted to the PICU from the ages of 3 months to 25 years, who had a predicted discharge >48 h from PICU admission, and who had a urinary catheter placed by time of first scheduled urine collection and in place for at least first 48 h of PICU admission. Patients with a predicted discharge after 48 h were identified in the admission progress note (prediction discharge date is estimated by the attending ICU provider as part our PICU daily clinical routine). Patients with history of end-stage renal disease and those immediately following renal transplant were excluded from the study. The CCHMC PICU is a tertiary care facility, admitting critically ill patients from all medical subspecialties, following elective and emergent surgery and following trauma, and from one of the busiest pediatric emergency department in North America.
Data Timing
Data were collected from first calendar day of PICU admission (Day0) for seven consecutive days until 7 days after admission (Day7). A minimum of 8 h from PICU admission constituted Day0. Urinary biomarkers were assessed the day after PICU admission, between 12 and 24 h after time of admission (Day1). Day3 consisted of the time period between 72 and 96 h after PICU admission.
Collected variables
Demographic information, admission diagnoses, comorbidities, height, weight, available laboratory values, vital signs and Pediatric Risk of Mortality III score (PRISM-III) [27] were collected at the time of admission. Baseline SCr was established as the lowest creatinine up to 3 months before PICU admission on Day0. Daily collected variables were assessed at 8:00 am for each patient on Days1–7 and included vital signs, laboratory values, nephrotoxin use, vasopressor use, mechanical ventilation support level and total ICU fluid intake and output.
Calculated variables and RAI
The RAI score was determined between 8 and 12 h from the time of PICU admission on Day0 as previously described [25]. Determination of Day0-RAI required the calculation of percent fluid overload (%FO) [28] from ICU admission and change in creatinine from baseline. If no baseline SCr was available in the computer system, a reference estimated creatinine clearance of 120 mL/min/m2 was used and a baseline creatinine was imputed using the patient's height in centimeters [29]. An RAI ≥8 was considered fulfillment of renal angina [25]. Fulfillment or the absence of renal angina was denoted ‘RA+’ or ‘RA−’.
Net ICU fluid balance was determined using simple subtraction of total fluid balance during ICU course. Urine output was calculated as mL/kg/h in 8 h blocks using PICU admission weight.
Outcomes
The primary outcome was the presence of severe AKI on Day3 (Day3-AKI). This persistent, severe AKI was classified as KDIGO AKI Stage 2 or 3 by change in SCr from baseline, consistent with our previous retrospective publications. Day3 was chosen since most PICU patients develop AKI within this timeframe, the time highlights the advantage of biomarkers on the PICU admission day to predict the outcome; in addition, Day3 is a clinically relevant time frame for AKI management. Secondary outcomes included Day7 and final kidney-specific organ dysfunction, including %FO, the presence of AKI and use of renal replacement therapy (RRT). Tertiary outcomes included ICU-specific endpoints such as duration of mechanical ventilation, length of stay (LOS), organ failure days and incidence of mortality.
Urine collection and biomarker analysis
Urine samples were collected on all enrolled patients. Urine was centrifuged and stored in aliquots at −80°C until measurement. Day1 biomarker values were used for the purposes of this initial analysis (urine obtained from Days1–4 when possible). uNGAL was assayed using a human-specific commercially available enzyme-linked immunosorbent assay (ELISA, AntibodyShop, Grusbakken, Denmark). uIL-18 and uL-FABP were measured using commercially available ELISA kits (Medical and Biological Laboratories Co., Nagoya, Japan and CMIC Co., Tokyo, Japan, respectively) as per manufacturer's instructions. The uKIM-1 ELISA was constructed using commercially available reagents (R&D Systems, Inc., Minneapolis, MN, USA). Optimal cutoff values for biomarkers were determined using sensitivity analysis and Youden's index for the prediction of Day3-AKI (for each biomarker tested individually) [26, 30].
Statistical analysis
All statistical analyses were performed using SigmaStat version 12.3 software (San Antonio, TX, USA), SAS version 9.2 (SAS Institute, Cary, NC, USA) and R version 2.14.1 (R Development Core Team, Vienna, Austria). R packages pROC and PredictABEL were used [31, 32]. Continuous variables were reported as median with interquartile range and compared using the Mann–Whitney test. Categorical variables were summarized using frequency and proportion and compared by χ2 or Fisher's exact tests. An RAI cutoff of ≥8 was used to define renal angina fulfillment (RA+) and this cutoff was used for operative characteristics [25]. Simple and multivariable logistic models were used to risk-stratify patients for Day3-AKI using RAI and its combinations with AKI biomarker(s) concentration levels for the prediction of Day3-AKI. AUC-ROC values were calculated for each prediction model (RAI and biomarker concentrations used as continuous variables) and compared using DeLong's method [33]. The Akaike Information Criterion (AIC), net reclassification improvement (NRI) and integrated discrimination improvement (IDI) quantified additional changes in discrimination after incorporation of AKI biomarkers [34, 35]. No risk categories were selected for the calculation of NRI. R package Hmisc was used for the calculation of NRI and IDI. Sample size was initially projected in multiple ways. In designing the study, we determined size based on the requirements of a reliable logistic model. A priori, we assumed four variables would be included in a comparative prediction model—the RAI index, age, PRISM-II score and the presence of sepsis. Ten events per variable means we needed 40 events (patients with AKI). Assuming a 10% incidence rate based on existing epidemiology reports, 400 patients needed to be enrolled. On initiation of the study, we had already begun to plan the multi-center AWARE study (NCT01987921) and realized we would not have 2 years to complete this pilot study. Based on our previous studies of RAI, we estimated that between 150 and 200 patients provided a large enough sample size to perform our analysis. In all analyses, a P-value of <0.05 was considered statistically significant.
RESULTS
Patients
A total of 184 consecutive patients were enrolled for study and all were used to derive demographic and outcome data; 156 (84.7%) remained in the ICU on Day3. Their data were used for Day3-AKI analyses. Of the 156, Day1 urinary biomarkers were measured in 114 patients representing 62% of the original sample (114/184).
Renal angina occurs frequently in a heterogeneous patient population and is associated with poor outcome
Approximately one in three patients (32.6%) was RA+ within the first 12 h of PICU admission. These patients were younger, had a higher prevalence of sepsis or history of transplant, and higher PRISM-III scores (Table 1). Additionally, RA+ on Day0 was associated with a longer duration of mechanical ventilation, greater number of organ failure days and higher mortality.
Table 1.
Demographics and outcomes of patients stratified by Day0 renal angina fulfillment
| Category | Overall | RA− | RA+ | P-value (RA+ versus RA−) | |
|---|---|---|---|---|---|
| Demographics | n (%) | 184 | 124 (67.4) | 60 (32.6) | — |
| Male (%) | 98 (52.7) | 63 (50.8) | 34 (56.7) | ns | |
| Age on admission (years) | 7.7 (2.7, 14.9) | 11.1 (3.1, 15.9) | 4.6 (1.9, 12.9) | 0.03 | |
| Weight (kg) | 27.2 (13.4, 53.9) | 31.4 (14.1, 54.9) | 17.4 (11.9, 49.3) | 0.06 | |
| Height (cm) | 127 (88, 158) | 130 (93, 159) | 106 (82, 152) | 0.05 | |
| BSA (m2) | 0.99 (0.57, 1.55) | 1.05 (0.61, 1.55) | 0.71 (0.52, 1.53) | 0.06 | |
| PRISM-III | 7 (3, 13.8) | 5 (2, 9) | 14 (7.3, 21) | <0.001 | |
| Admitted from | |||||
| Surgical post-op | 77 (41.2) | 65 (52.4) | 12 (20.0) | ||
| ED | 53 (28.8) | 30 (24.2) | 23 (38.3) | ||
| General Peds | 18 (9.6) | 12 (9.7) | 6 (10.0) | ||
| Transport | 14 (7.5) | 6 (4.8) | 8 (13.3) | ||
| Liver/GI | 16 (8.6) | 7 (5.6) | 9 (15.0) | ||
| Heme-Onc | 4 (2.1) | 4 (3.2) | 0 (0) | ||
| BMT | 2 (1.1) | 0 (0) | 2 (3.3) | ||
| Sepsis Dx (%) | 45 (24.5) | 20 (16.1) | 25 (41.7) | χ 2 = 14.6, P < 0.001 | |
| Transplant history (%) | 21 (11.4) | 6 (4.8) | 15 (25) | χ 2 = 16.1, P < 0.001 | |
| Outcomes | Mechanical Vent days | 2 (0, 6) | 1 (0, 5) | 3 (1, 6.8) | 0.04 |
| CRRT use (%) | 6 (3.3) | 1 (0.8) | 5 (8.3) | χ 2 = 6.9, P = 0.009 | |
| Organ failure days | 2 (0, 7) | 1 (0, 6) | 4 (1, 8) | 0.003 | |
| Hospital LOS (days) | 14 (8, 30) | 14 (8, 29) | 16 (8, 41) | 0.08 | |
| ICU LOS (days) | 5 (3, 11) | 4 (3, 11) | 6 (4, 13) | 0.19 | |
| ICU mortality (%) | 12 (6.5) | 1 (0.8) | 11 (18.3) | χ 2 = 17.1, P < 0.001 |
Patients in AKI-CHERUB dichotomized by the fulfillment or the absence of renal angina (RA+ or RA−) on the day of ICU admission. All values are expressed as medians with interquartile ranges except where designated. n, sample size; BSA, body surface area; ED, emergency department; General Peds, general pediatric ward; GI, gastrointestinal; Heme-Onc, hematology-oncology; BMT, bone marrow transplant unit; Dx, diagnosis; Vent, ventilation; CRRT, continuous renal replacement therapy; ns, not significant.
Fulfillment of renal angina on admission is associated with an increased incidence of subsequent, severe AKI
In the 156 patients remaining in the ICU on Day3, 52 (33.3%) were RA+ on Day0. Day3-AKI incidence was 9.6% (15/156); 12/15 (80%) of Day0 RA+ were AKI+ on Day3 (Table 2). Day0 RA+ status was associated with a greater incidence of Day3-AKI than Day0 RA− status (23.1 versus 2.9%, χ2 = 16.3, P < 0.001). Additionally, Day0 RA+ patients carried significantly greater positive net fluid balance and higher %FO on Day3 than RA− patients.
Table 2.
Day3 kidney health and ICU outcomes stratified by renal angina
| Category | All patients | Day0 RA− | Day0 RA+ | P-value (RA− versus RA+) | |
|---|---|---|---|---|---|
| Demographics | n (%) | 156 | 104 (66.6) | 52 (33.3) | — |
| Male (%) | 82 (52.6) | 52 (50) | 30 (57.7) | ns | |
| Age (years) | 8.4 (2.7, 15.5) | 11.7 (2.8, 16.0) | 5 (2.0, 13.9) | 0.13 | |
| Day0 PRISM-III | 8 (3, 14) | 5 (2, 10) | 14 (8.3, 20) | <0.001 | |
| Day3 kidney health | AKI by SCr-KDIGO 2–3 | 15 (9.6) | 3 (2.9) | 12 (23.1) | χ 2 = 16.3, P < 0.001 |
| Net fluid balance (mL) | 2311 (613, 4333) | 1831 (485, 3904) | 2768 (1040, 5026) | 0.03 | |
| %FO | 9.3 (3.2, 15.3) | 6.8 (2.6, 15.3) | 11.8 (6.8, 15.7) | 0.009 | |
| ICU outcomes | Mechanical Vent days | 2 (0, 7) | 1.5 (0, 6) | 3.5 (1, 7.8) | 0.05 |
| CRRT use (% group) | 6 (3.8) | 1 (1) | 5 (9.6) | χ 2 = 4.8, P = 0.03 | |
| Organ failure days | 3 (1, 8) | 2 (0, 7) | 6 (2, 8) | 0.007 | |
| Hospital LOS (days) | 17 (8, 34) | 16 (8, 29) | 21 (9, 47) | 0.20 | |
| ICU LOS (days) | 6 (3, 12) | 5 (3, 11) | 7 (4, 16) | 0.19 | |
| Mortality (% group) | 9 (5.7) | 1 (1) | 8 (15.4) | χ 2 = 17.1, P < 0.001 |
Patients in AKI-CHERUB remaining in the ICU on Day3 of admission to the PICU dichotomized by the fulfillment or the absence of renal angina (RA+ or RA−) on the day of ICU admission (Day0). All values are expressed as medians with interquartile ranges except where designated. n, sample size; AKI by SCr-KDIGO 2–3, AKI diagnosed by change in SCr from baseline as per the KDIGO staging criteria Stages 2–3; Vent, ventilation; CRRT, continuous renal replacement therapy; ns, not significant.
The RAI is independently associated with risk of Day3 – AKI
Multivariate logistic regression performed on the univariate day of admission variables associated with Day3-AKI identifies high odds ratios for severe injury with higher severity of illness score (PRISM-III), the presence of sepsis, history of transplant and renal angina on admission (Table 3).
Table 3.
Multivariate regression for Day3 AKI
| Criterion | Odds ratio |
|
|---|---|---|
| All Day0 patients | Patients with Day3 data | |
| PRISM-III | 1.12 (1.05, 1.19) | 1.09 (1.04, 1.16) |
| Sepsis | 7.1 (2.26, 22.3) | 7.6 (2.44, 23.7) |
| Transplant history | 8.62 (2.69, 27.6) | 9.63 (3.04, 30.5) |
| Renal angina | 10.1 (2.71, 37.7) | 10.1 (2.7, 37.3) |
Multivariate regression analysis for Day3-AKI as predicted by the criteria with univariate association on admission (P < 0.10). Criterion included PRISM-III score, diagnosis of sepsis, history of transplant and the fulfillment of renal angina on Day0. Results expressed with 95% CI.
Day3-AKI is associated with poor outcome in a heterogeneous population of critically ill children
Day3-AKI was associated with ICU mortality (Table 4). Day3-AKI increased duration of ICU LOS, prolonged mechanical ventilation and was associated with a greater number of organ failure days than the absence of AKI. Similar associations with worse longer term outcomes are seen when patients remaining in the ICU on Day3 are dichotomized by Day0 ‘±' RA− or ‘±’ Day3-AKI (Tables 2 and 4).
Table 4.
Outcomes stratified by Day3-AKI
| Category | Overall | No AKI | AKI | P-value (no AKI versus AKI) | |
|---|---|---|---|---|---|
| Demographics | n (%) | 156 | 141 (90.4) | 15 (9.6) | — |
| Male (%) | 98 (52.7) | 71 (50.4) | 11 (73.3) | ns | |
| Age (years) | 8.4 (2.7, 15.5) | 8.2 (2.7, 15.1) | 12.8 (2.9, 18.0) | ns | |
| Day0 PRISM-III | 8 (3, 14) | 7 (3, 13) | 15 (11, 23) | <0.001 | |
| Day0 renal angina | 52 (33.3) | 40 (28.4) | 12 (80) | χ 2 = 16.2, P < 0.001 | |
| Sepsis Dx (%) | 41 (26.3) | 31 (21.9) | 10 (66.7) | χ 2 = 13.9, P < 0.001 | |
| Transplant history (%) | 20 (12.8) | 13 (9.2) | 7 (46.7) | χ 2 = 17.1, P < 0.001 | |
| Outcomes | Mechanical Vent days | 2 (0, 7) | 2 (0, 6) | 6 (3, 15) | 0.03 |
| CRRT use (%) | 6 (85.7) | 0 | 6 (100) | χ 2 = 48.8, P < 0.001 | |
| Organ failure days | 3 (1, 8) | 3 (1, 7) | 7 (3, 16) | 0.03 | |
| Hospital LOS (days) | 17 (8, 34) | 16 (8, 30) | 38 (16, 69) | 0.02 | |
| ICU LOS (days) | 6 (3, 12) | 6 (3, 12) | 8 (6, 18) | 0.02 | |
| Mortality (%) | 9 (7.1) | 6 (4) | 3 (20) | χ 2 = 7.4, P = 0.007 |
Patients in AKI-CHERUB remaining in the ICU on Day3 of admission to the PICU dichotomized by the presence or the absence of severe AKI on Day3 of ICU admission. All values are expressed as medians with interquartile ranges except where designated. n, sample size; AKI by SCr-KDIGO 2–3, AKI diagnosed by change in SCr from baseline per the KDIGO Staging Criteria Stages 2–3; Dx, diagnosis; Vent, ventilation; CRRT, continuous renal replacement therapy; LOS, length of stay; ns, not significant.
Incorporation of urinary biomarkers into the RAI model optimizes Day3-AKI prediction
Biomarker incorporation into the RAI significantly increased the specificity for severe AKI on Day3. Measured on the day after admission (Day1), NGAL, KIM-1 and IL-18 increased the specificity and likelihood for severe AKI and also demonstrated NRI (Table 5). Notably, after inclusion of NGAL in the model, the AUC-ROC of Day0-RAI to predict Day3-AKI increased from 0.80 [95% confidence interval (CI): 0.58, 1.00] to nearly unity (0.97, 95% CI: 0.93, 1.00), decreased the AIC and demonstrated significant NRI (1.64, P < 0.001) and IDI (0.419, P = 0.02). Odds ratios for the development of Day3-AKI were calculated for biomarkers in combination with the RAI for Day3-AKI (Table 5) but for NGAL positivity in the context of renal angina fulfillment was not calculable because of a non-existent negative event rate. Similarly, no statistical analysis was possible on biomarker prediction of Day3-AKI in patients without Day0 renal angina given the low event rate.
Table 5.
Discrimination for Day3-AKI by the RAI in combination with urinary biomarkers
| Terms | Sensitivity | Specificity | PPV | NPV | +LR | ORa (95% CI) | AUC-ROC (95% CI) | AIC | NRI (P-value) | IDI (P-value) |
|---|---|---|---|---|---|---|---|---|---|---|
| Day0 RAI | 80 (52–97) | 72 (64–78) | 20 (11–32) | 98 (93–100) | 2.8 (2.0–4.0) | n/a | 0.80 (0.58, 1.00) | 48.8 | n/a | n/a |
| Day0 RAI+ NGAL | 86 (42–99) | 85 (77–90) | 23 (9–44) | 99 (95–100) | 5.6 (3.4–9.2) | NCb | 0.97 (0.93, 1.00) | 28.5 | 1.64 (<0.001) | 0.42 (0.02) |
| Day0 RAI+ KIM-1 | 43 (10–82) | 95 (90–98) | 33 (8–70) | 97 (92–99) | 9.3 (2.9–30) | 5.1 (0.5, 50.4) | 0.77 (0.53, 1.00) | 48.8 | 0.69 (0.007) | 0.039 (0.12) |
| Day0 RAI+ L-FABPc | 86 (57–98) | 56 (58–64) | 15 (8–25) | 98 (92–100) | 1.9 (1.5–2.6) | 2.5 (0.2, 43.2) | 0.82 (0.69, 0.95) | 82.0 | 0.49 (0.04) | 0.04 (0.31) |
| Day0 RAI+ IL-18c | 57 (29–82) | 97 (92–99) | 62 (32–86) | 96 (91–99) | 17.4 (6.6–46) | 5.5 (0.6, 47.1) | 0.79 (0.65, 0.92) | 81.1 | 0.87 (0.001) | 0.05 (0.05) |
Predictive performance and discrimination was determined for the primary outcome: severe AKI diagnosed by KDIGO Stage 2–3 change of creatinine from baseline. Sensitivity, specificity, PPV, NPV, positive likelihood ratio (+LR), AUC-ROC, change in AIC, NRI and IDI were performed on the RAI on Day0 of admission to the PICU. Effect of including urinary biomarkers NGAL, KIM-1, L-FABP and IL-18 measured on Day1 of PICU admission was then determined. Cutoff values used for the biomarkers were determined based on optimal Youden's index results on individual urinary biomarkers for the population tested (in ng/mL): NGAL >150, KIM-1 >4000, l-FABP >150 and IL-18 >1000. Patients included in this analysis: n = 137. Results expressed with 95% CI. n/a, not available.
aOdds ratio—analysis done for impact of biomarkers in renal angina positive patients.
bNot calculable.
c167.
DISCUSSION
In this study, we prospectively validate the concept that provision of clinical context to direct biomarker testing enhances the ability to predict clinically significant AKI in critically ill children. In our earlier, retrospective derivation and validation studies [25, 26], the advantage of the RAI methodology for AKI risk stratification was clear: the RAI is easily calculable (versus severity of illness scores), early (on ICU admission day) and offers comparable or superior AKI risk stratification versus traditional markers of illness or patient demographics. Another important difference from our previous retrospective analyses of RAI [26] is that CHERUB incorporated urinary biomarkers. Prospective testing in patients for the specific purpose of analyzing AKI prediction demonstrates incorporation of uNGAL into the RAI model predicts severe and persistent AKI with an AUC-ROC of 0.97. ‘CHERUB’ models a relevant, clinical time course: a critically ill child is admitted to the ICU, resuscitated and stabilized over a period of 8–12 h. The RAI can then be used to rule in risk of AKI, triggering measurement of a highly specific AKI biomarker for the prediction of subsequent severe AKI. The timing of this predictive model, within 8–12 h of admission for nearly all patients, could be used in a targeted trial examining AKI management involving hemodynamic interventions, nephrotoxic medications and targets for fluid balance (Figure 2).
FIGURE 2:
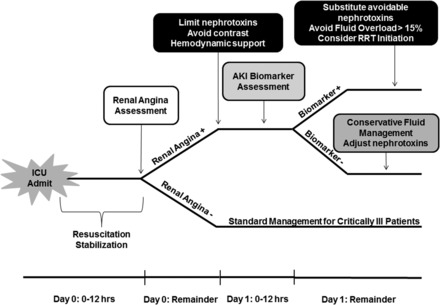
Schema depicted represents a potential trial of prospective evaluation on outcome based on use of the RAI for AKI risk stratification after admission to the ICU.
The RAI risk stratifies patients for subsequent, severe AKI—an injury at a point in time of ICU admission with significant clinical consequence. As our data demonstrate, Day3-AKI is associated with worse clinical outcomes including prolonged LOS, longer time on mechanical ventilation and increased rate of death. This novel finding supports our a priori hypothesis in both the derivation/validation studies of RAI and CHERUB, that AKI on Day3 is a clinically relevant marker of illness and disease trajectory. Severity of illness scores such as PRISM-III and the Pediatric Logistic Organ Dysfunction score in pediatrics and adult severity of illness are not calibrated for severity of illness outside of time of admission [24]. Creation of complex models for AKI risk stratification or prediction to be used in real-time is cumbersome and not practical for bedside care. Traditional ‘AKI prediction’ scores such as the Lianos, Bullocks, Mehta and SHARF 2 scores work well for population study but are not feasible for individual patient analysis. The RAI is designed to be a practical, bedside tool to assess whether a patient is, or is not at-risk for developing AKI.
Optimized biomarker assessment can significantly impact time to therapeutic intervention and associated outcome. Mortality from ACS began to decline with the advent of both care units dedicated to cardiac patients and a diagnostic biomarker test with high discrimination for injury in patients demonstrating cardiac angina. The modern ACS gold standard biomarker, cTi, loses specificity when measured in patients without risk factors or signs of illness. Fortunately for those patients, heart attack recognition has a clinical advantage over patients suffering from kidney attack [8]. Simply stated, AKI does not ‘hurt’. We suggest, however, that renal angina is easily identifiable and offers diagnostic direction for biomarker use that parallels chest pain for troponin. The RAI is a risk discrimination model that optimizes the pre-test probability of disease. Biomarkers tested using this context then have an optimized post-test probability of detecting AKI. Just as indiscriminate use of troponin leads to overall poor prediction of the ACS, capricious testing of AKI biomarkers will lead to poor diagnostic performance and lessened cost-effectiveness, and will delay integration into routine bedside clinical practice. In patients without Day0 renal angina, there is very low risk of Day3-AKI (i.e. a very low probability of Day3-AKI) even with a positive biomarker test on Day1 (Supplementary Table S1). Judicious AKI biomarker assessment, using the context of patient risk, increases predictive performance and clinical efficacy, and offers hope of more expeditious acceptance into management algorithms [36, 37].
We report the highest discrimination of uNGAL for the prediction of severe AKI in the general critical care population in either adults or children. Outside of the original paper describing NGAL for AKI prediction after CPB (AUC-ROC 0.99), very few reports demonstrate such a high level of discrimination [15]. In a recent critical evaluation of NGAL performance in critical care and emergency department settings, the combined AUC-ROC for uNGAL in 5347 patients was 0.80 and for serum NGAL in 3154 was 0.79 [22]. Discrimination calculation using the AUC-ROC to test NGAL in patients without Day0 renal angina in our study was impossible due to the infrequency of Day3-AKI in this population [only 2.9% (3/104) of patients negative for Day0 renal angina had Day3-AKI compared with 23.1% (12/52) patients for positive for renal angina on Day0]. The performance characteristics of the RAI incorporating urinary biomarkers demonstrate increases in the specificity and positive likelihood ratios for the primary outcome of Day3-AKI (Table 5). These results support the argument that testing of a biomarker in a targeted, risk-stratified patient (i.e. renal angina positive) offers a real-time predictive advantage for a treating clinician.
Patients with early fulfillment of renal angina demonstrate a significantly higher degree of fluid accumulation in their subsequent ICU course. Because initial %FO is included in the calculation of the RAI (the worse of %FO or change from baseline SCr is used to denote injury score), separating early fluid accumulation from persistent severe fluid accumulation (‘fluid overload’) may be an important sub-analysis, particularly in patients with AKI. In the scope of this report, however, we suggest that regardless of initial fluid accumulation, higher net fluid balance and increased %FO in RA+ patients on Day3 may be a clinically important prognostic marker of outcome. In the context of oligo-anuric AKI requiring RRT, observational studies performed originally in pediatrics demonstrate that high %FO at the time of RRT initiation is strongly associated with poor outcome (each 1% in FO increases mortality by 3%) [38]. Outside of patients receiving renal support, association of positive net fluid balance with increased morbidity in mechanically ventilated patients has been highlighted in adults [39] and in several recent pediatric reports [40–42]. Additionally, significant fluid overload may actually mask the true incidence of AKI [43, 44]. Pooled data indicate that AKI diagnosed by both increases in SCr and oliguria has significantly worse outcomes than either alone [45].
There are several potential limitations to our study. Like many others, we describe the limitations of using changes in SCr as an early sign of injury (i.e. a risk stratification biomarker), particularly in children. However, these limitations exist using creatinine without stratified, objective patient context. The RAI is a creatinine-based risk stratification methodology derived and validated in a number of critically ill patient populations, novel because of the stratified, objective use of creatinine changes in patient context. Despite the excellent discrimination for Day3-AKI afforded by biomarker positivity in the context of renal angina positivity, the positive predictive value (PPV) remains low (15–62%). The reliance on PPV as a marker of statistical predictive efficacy should be limited, however, as the incidence of AKI in the cohort was 10%. In a population with higher AKI prevalence, e.g. critically ill septic children only, the PPV would be higher [26]. The practical use of the RAI is to rule out disease—and the very high negative predictive value (NPV) supports our suggestion that the index serves to increase the pre-test probability of disease. As a single-center study, management of patients is subject to the inherent bias of our institution. However, both the AUC of RAI for Day3-AKI and AKI incidence rate in this study were essentially identical to our previous derivation and validation studies of the RAI [25] and other epidemiology studies performed in tertiary PICU settings [46]. Given our sample size, our positive event rate (Day3-AKI) limited some of the statistical analyses (e.g. AUC). As listed in the Materials and methods, in the initial study design nearly 400 patients were targeted for enrollment and we had to curtail the study to initiate our broader, multi-center prospective observational study. Additionally, although we had over 180 patients enrolled in the study from the time of admission, many patients were transferred from the ICU earlier than anticipated and did not remain in the ICU on Day3 as was expected. Patient safety initiatives to reduce the rate of catheter-associated urinary tract infections were instituted in the middle of the study and impacted the rate of both patient enrollment and the ability to capture urine samples for biomarker testing. Although we collected biomarkers on Days2–4, this initial analysis investigated only the Day1 biomarkers. This selection was intentional as a feasible clinical workflow, described earlier, is illustrated in Figure 1. The temporal profile of the biomarkers in response to injury may be more clearly demonstrated with future analysis of Days2–4 urine specimens.
We conclude that the prediction of severe and persistent AKI produced by biomarkers analyzed in the context of renal angina fulfillment identifies the patients at the highest risk of injury. As we previously demonstrated, the high NPV of the RAI supports its use to rule out AKI. In this study, the ability to rule in AKI using novel biomarkers is optimized by a clinical model. While larger and multi-center studies of RAI and AKI biomarkers should be performed to bolster our findings, we suggest that the ease of use and performance of the RAI supports integration into clinical practice at this time.
AUTHORS’ CONTRIBUTIONS
S.M.: Co-Principal Investigator, primary data collection and analysis, manuscript editing; S.G.: Co-Principal Investigator, data analysis, manuscript editing; T.M.: data collection, entry and urinary specimen collection; T.T.: data collection, entry and urinary specimen collection; L.F.: Statistician; A.K.: data collection, analysis and urinary specimen collection; P.A.: data entry, urinary specimen collection; M.B.: urinary biomarker processing; R.B.: Co-Principal Investigator, data entry, analysis, manuscript preparation and editing.
CONFLICT OF INTEREST STATEMENT
The results presented in this paper have not been published previously in whole or in part, except in abstract format.
Supplementary Material
ACKNOWLEDGEMENTS
S.M. was sponsored in the Cincinnati Children's Hospital Medical Center's Acute Care Nephrology Fellowship through a grant from Gambro Renal Products, Inc. RedCap database support was provided from the Center for Clinical and Translational Science Training Grant Support (8UL1-TR000077). Urinary biomarker processing was supported by a Nephrology Center of Excellence Grant (P50 DK096418-01, Devarajan).
REFERENCES
- 1. Susantitaphong P, Cruz DN, Cerda J et al. . World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 2013; 8: 1482–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thomas ME, Blaine C, Dawnay A et al. . The definition of acute kidney injury and its use in practice. Kidney Int 2015; 87: 62–73 [DOI] [PubMed] [Google Scholar]
- 3. Akcan-Arikan A, Zappitelli M, Loftis LL et al. . Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 2007; 71: 1028–1035 [DOI] [PubMed] [Google Scholar]
- 4. Group KDIGOKAKIW. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012; 2: 1–138 [Google Scholar]
- 5. Hoste EA, Clermont G, Kersten A et al. . RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care 2006; 10: R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mehta RL, Kellum JA, Shah SV et al. . Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11: R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewington AJ, Cerda J, Mehta RL. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int 2013; 84: 457–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kellum JA, Bellomo R, Ronco C. Kidney attack. J Am Med Assoc 2012; 307: 2265–2266 [DOI] [PubMed] [Google Scholar]
- 9. Himmelfarb J, Joannidis M, Molitoris B et al. . Evaluation and initial management of acute kidney injury. Clin J Am Soc Nephrol 2008; 3: 962–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Devarajan P. Biomarkers for the early detection of acute kidney injury. Curr Opin Pediatr 2011; 23: 194–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meersch M, Schmidt C, Van Aken H et al. . Validation of cell-cycle arrest biomarkers for acute kidney injury after pediatric cardiac surgery. PLoS One 2014; 9: e110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krawczeski CD, Goldstein SL, Woo JG et al. . Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol 2011; 58: 2301–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parikh CR, Devarajan P, Zappitelli M et al. . Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol 2011; 22: 1737–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zappitelli M, Krawczeski CD, Devarajan P et al. . Early postoperative serum cystatin C predicts severe acute kidney injury following pediatric cardiac surgery. Kidney Int 2011; 80: 655–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mishra J, Dent C, Tarabishi R et al. . Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005; 365: 1231–1238 [DOI] [PubMed] [Google Scholar]
- 16. Ronco C, Legrand M, Goldstein SL et al. . Neutrophil gelatinase-associated lipocalin: ready for routine clinical use? An international perspective. Blood Purif 2014; 37: 271–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vanmassenhove J, Vanholder R, Nagler E et al. . Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrol Dial Transplant 2013; 28: 254–273 [DOI] [PubMed] [Google Scholar]
- 18. Kashani K, Al-Khafaji A, Ardiles T et al. . Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013; 17: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pickering JW, Endre ZH. The clinical utility of plasma neutrophil gelatinase-associated lipocalin in acute Kidney Injury. Blood Purif 2013; 35: 295–302 [DOI] [PubMed] [Google Scholar]
- 20. Cruz DN, de Cal M, Garzotto F et al. . Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med 2010; 36: 444–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wheeler DS, Devarajan P, Ma Q et al. . Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med 2008; 36: 1297–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haase-Fielitz A, Haase M, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status. Ann Clin Biochem 2014; 51(Pt 3): 335–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lim W, Whitlock R, Khera V et al. . Etiology of troponin elevation in critically ill patients. J Crit Care 2010; 25: 322–328 [DOI] [PubMed] [Google Scholar]
- 24. Basu RK, Chawla LS, Wheeler DS et al. . Renal angina: an emerging paradigm to identify children at risk for acute kidney injury. Pediatr Nephrol 2012; 27: 1067–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Basu RK, Zappitelli M, Brunner L et al. . Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int 2014; 85: 659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Basu RK, Wang Y, Wong HR et al. . Incorporation of biomarkers with the renal angina index for prediction of severe AKI in critically ill children. Clin J Am Soc Nephrol 2014; 9: 654–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated pediatric risk of mortality score. Crit Care Med 1996; 24: 743–752 [DOI] [PubMed] [Google Scholar]
- 28. Goldstein SL, Currier H, Graf C et al. . Outcome in children receiving continuous venovenous hemofiltration. Pediatrics 2001; 107: 1309–1312 [DOI] [PubMed] [Google Scholar]
- 29. Zappitelli M, Parikh CR, Akcan-Arikan A et al. . Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol 2008; 3: 948–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Basu RK, Wong HR, Krawczeski CD et al. . Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J Am Coll Cardiol 2014; 64: 2753–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robin X, Turck N, Hainard A et al. . pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011; 12: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kundu S, Aulchenko YS, van Duijn CM et al. . PredictABEL: an R package for the assessment of risk prediction models. Eur J Epidemiol 2011; 26: 261–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–845 [PubMed] [Google Scholar]
- 34. Pencina MJ, D'Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 2011; 30: 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pencina MJ, D'Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med 2010; 48: 1703–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goldstein SL, Chawla LS. Renal angina. Clin J Am Soc Nephrol 2010; 5: 943–949 [DOI] [PubMed] [Google Scholar]
- 37. Ronco C, Ricci Z. The concept of risk and the value of novel markers of acute kidney injury. Crit Care 2013; 17: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sutherland SM, Zappitelli M, Alexander SR et al. . Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis 2010; 55: 316–325 [DOI] [PubMed] [Google Scholar]
- 39. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network, Wheeler AP, Bernard GR et al. . Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 2006; 354: 2213–2224 [DOI] [PubMed] [Google Scholar]
- 40. Hazle MA, Gajarski RJ, Yu S et al. . Fluid overload in infants following congenital heart surgery. Pediatr Crit Care Med 2013; 14: 44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Valentine SL, Sapru A, Higgerson RA et al. . Fluid balance in critically ill children with acute lung injury*. Crit Care Med 2012; 40: 2883–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arikan AA, Zappitelli M, Goldstein SL et al. . Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med 2012; 13: 253–258 [DOI] [PubMed] [Google Scholar]
- 43. Macedo E, Bouchard J, Soroko SH et al. . Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care 2010; 14: R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Basu RK, Andrews A, Krawczeski C et al. . Acute kidney injury based on corrected serum creatinine is associated with increased morbidity in children following the arterial switch operation. Pediatr Crit Care Med 2013; 14: e218–e224 [DOI] [PubMed] [Google Scholar]
- 45. Kellum JA, Sileanu FE, Murugan R et al. . Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol 2015; 26: 2231––2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schneider J, Khemani R, Grushkin C et al. . Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med 2010; 38: 933–939 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


