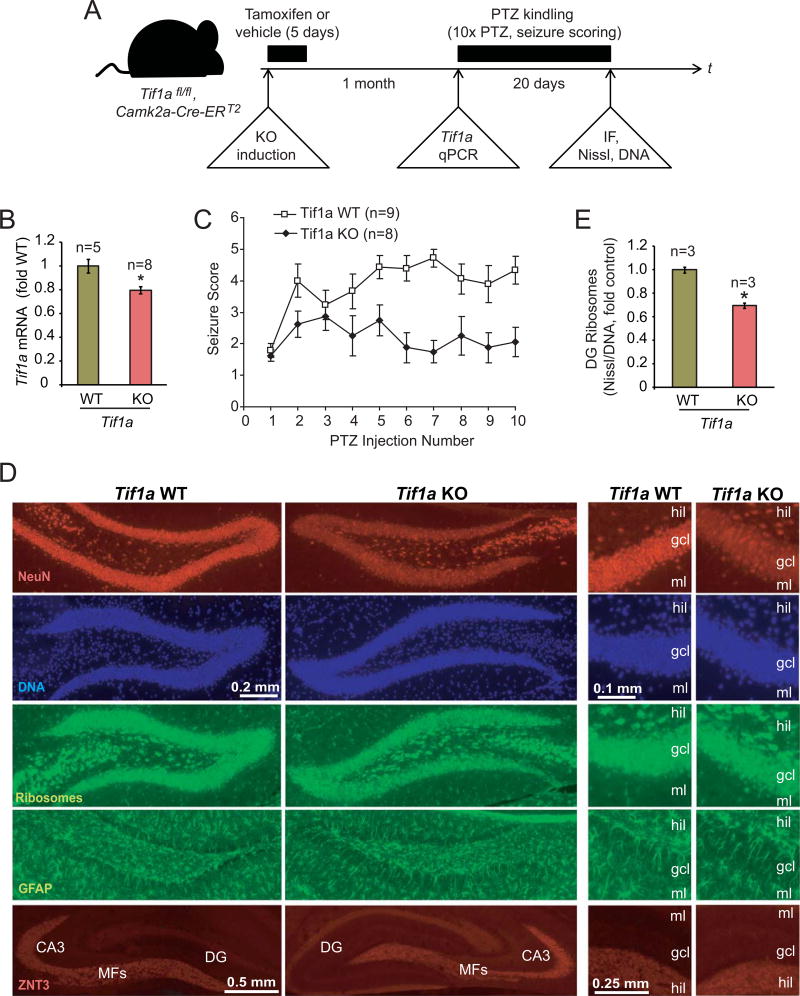
Figure 5. PTZ kindling is disrupted after deletion of the Pol1 co-activator Tif1a from excitatory forebrain neurons.
A, The experimental design. Tif1afl/fl, Camk2a-Cre-ERT2 mice were treated for 5 days with tamoxifen to induce Tif1a KO selectively in excitatory forebrain neurons. After a month, some animals were killed and hippocampal Tif1a mRNA expression was determined. Other animals were used for a pilot experiment with an acutely pro-convulsive dose of PTZ (60 mg/kg) to verify unaffected PTZ responsiveness of KO mice (Table 2). The rest of animals was subjected to a kindling paradigm that consisted of 10 i.p. injections of PTZ at a low dose of 35 mg/kg every other day; PTZ-induced seizures were scored using modified Racine scale. Then, animals were killed and structure of the dorsal hippocampus was analyzed by immunofluorescence (IF) or FluoroJade-B staining as well as ribosome (NeuroTrace Green, Nissl) and DNA (Hoechst-33258) co-stainings. B, One month after tamoxifen treatment, Tif1a mRNA levels were reduced in hippocampi of KO animals as revealed by qRT-PCR; Gapdh was used as a normalizer; the control group (WT) consisted of vehicle-treated Tif1afl/fl, Camk2a-Cre-ERT2 mice. C, Seizure scores of PTZ kindled mice. Note increasing seizure responses (i.e. kindling) in WT but not KO mice. The 2-way repeated measure ANOVA revealed significant effects of genotype (F1/15= 14.79, p=0.00159), number of injections (F9/135=3.85, p=0.000232), and the interaction between genotype and the number of injections (F9/135=2.987, p=0.002823). The KO group included 6 males and 2 females; the WT group included tamoxifen-treated Tif1afl/fl mice (3 males) as well as vehicle-treated Tif1afl/fl, Camk2a-Cre-ERT2 mice (4 males and 2 females). D, Representative images of immunostainings for the neuronal marker NeuN, the mossy fiber marker Znt3, the reactive astrocyte marker GFAP, and co-stainings of ribosomes and DNA. No major structural changes or reactive gliosis in the DG of the PTZ-kindled KO mice were revealed; similarly, no changes were observed in other forebrain regions (not shown). Only a small increase in FluoroJade-B-positive cells was noted in the DG but not the CA region indicating no major neurodegenerative pathology in the hippocampus of the Tif1a KO mice (Table 3, Supplementary Fig. S2). The moderate, KO-associated decrease in NeuN staining intensity in granule cell layer (gcl) appears to be caused by lower cellular levels of NeuN signal rather than reduced number of NeuN-positive cells; MFs, mossy fibers; hil, DG hilus; gcl, DG granule cell layer; ml, DG molecular layer. E, DNA-normalized content of ribosomes was determined in the gcl by dividing fluorescence intensity of ribosome signal by that of DNA in double stained sections. A moderate decrease of the DG ribosome content was present in Tif1a KO mice. Data represent averages ± SEM; *, p<0.05 (u-test).