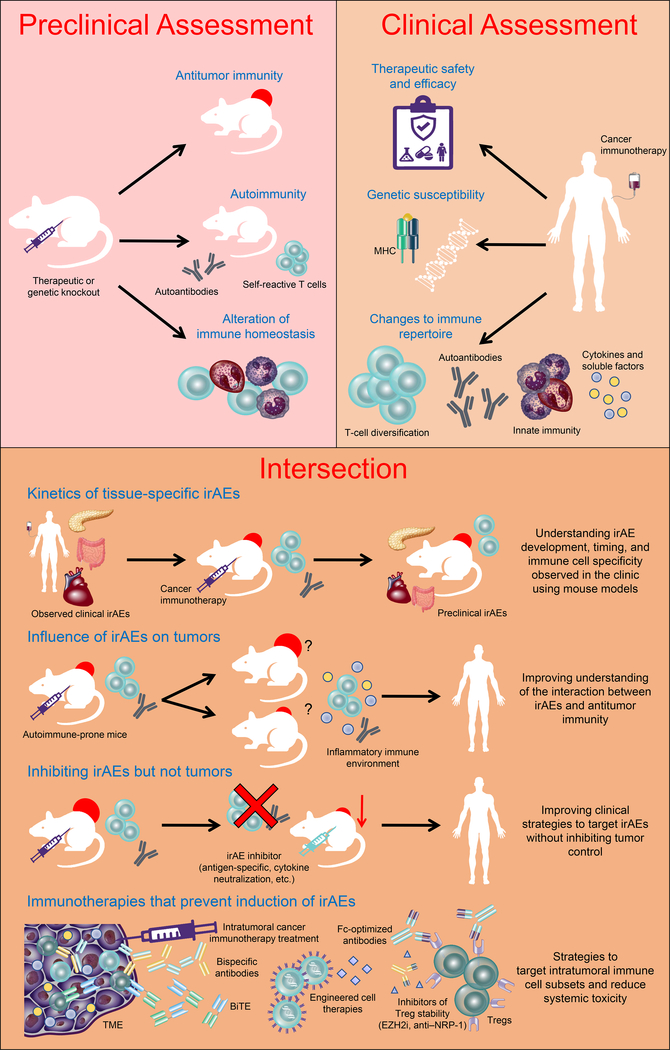
Figure 1. The intersection between preclinical and clinical studies.
Cancer immunotherapies undergo preclinical screening for their potential suitability for clinical use, with considerations to their ability to mediate antitumor immunity and autoimmunity determined. Following translation to clinical utility, the ability to answer clinical questions relating to response to therapy and safety profile is critical for clinical success. Many of these questions cannot be answered using available clinical parameters alone and rely on revisiting preclinical models. This represents an intersection between preclinical and clinical studies to improve our understanding of therapy-induced phenomena, such as the development of irAEs, to improve mechanistic understanding and clinical management. Ag: antigen; BiTE: Bispecific T-cell engager; EZH2i: enhancer of zeste homolog 2 inhibitor; irAEs: immune-related adverse events; MHC: major histocompatibility complex; NRP1: neuropilin 1; TME: tumor microenvironment; Treg: regulatory T cell.