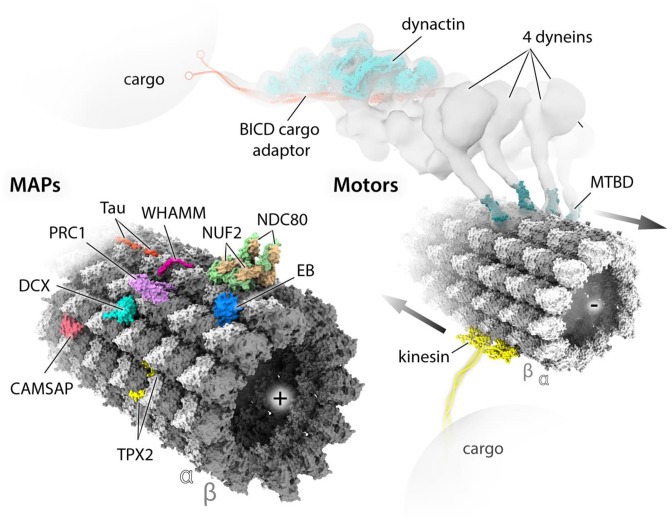
Figure 2. MAPs and motor proteins studied by cryo-EM.
Several structures of MTBDs or MT-binding polypeptide regions of MAPs and motor proteins have been determined in association with MT lattice by single-particle cryo-EM performed on decorated MTs. Atomic models of these structures are placed at relevant sites on the surface of an atomic model of MT lattice, all shown in solvent-excluded surfaces (SES) representation generated with ChimeraX [71]. The MT model was obtained by fitting a 6-dimer atomic model (PDB: 6EVZ) into near-atomic resolution cryo-EM map (EMDB: EMD-3964); the polarity of each MT is indicated by (+) and (−) signs. MAP models include: tau (PDB: 6CVN), WHAMM (PDB: 5X1G), NDC80/NUF2 (PDB: 3IZ0), EB (PDB: 3JAK), PRC1 (PDB: 5KMG), DCX (PDB: 4ATU), CAMSAP (PDB: 5M5C) and TPX2 (PDB: 6BJC). Motor models include two copies of an example typical (+) end directed kinesin motor domain (PDB: 5ND7) and four copies of the (−) end directed dynein MTBD (PDB: 3J1T) reconstructed together with MTs. The cluster of four dyneins assembled on dynactin and the MT was identified by cryo-ET [66], with lower resolution than the single-particle studies, which is indicated with isosurface representation of the experimental density (EMDB: EMD-7001). Structures of all dynein domains have been determined at high-resolution by both X-ray and cryo-EM studies not involving MT templates/tracks, hence not fitted into the density, except the dynactin/BICD part of the complex indicated for clarity. Kinesin motor domains are joined (dimerized) by the cartoon of a kinesin neck region and coiled-coil stalk region extending to cargo-binding elements. Similarly, BICD cargo adaptor is extended with a cartoon of cargo-binding elements.