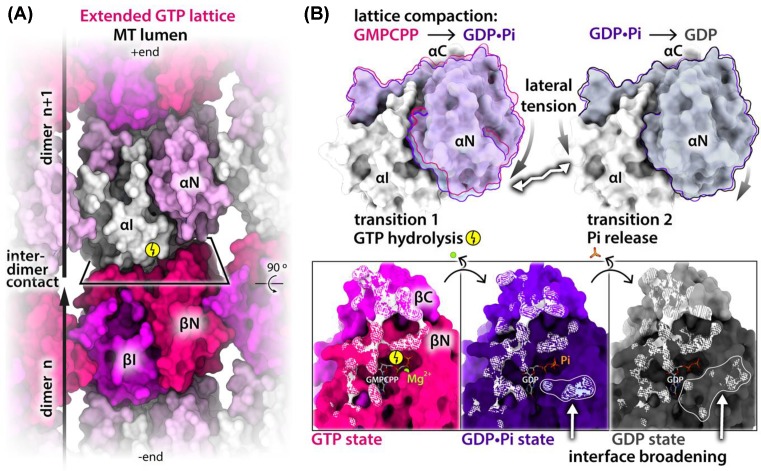
Figure 4. Uneven compression of α-tubulin and the resultant strengthening of longitudinal and weakening of lateral lattice contacts.
(A) MT extended lattice fragment (PDB: 6EVW) seen from the lumen and colored by tubulin subdomains: αI, βI, αN, βN–intermediate and N-terminal domains of tubulin α and β subunits, respectively. Tubulin dimer boundaries and lattice polarity are indicated, and longitudinal inter-dimer interface is framed in a perspective rectangle; C domains are not visible from this view as they project from the outside of the MT wall. (B) Top, αN subdomain movements after GTP hydrolysis (transition 1) and after Pi release (transition 2) relative to the αI subdomain; αN in the GDP.Pi state is semi-transparent blue and in the GDP state—gray. Outlines are included for convenient inspection of the movements; αN moves further than αI, as denoted with different sizes of the curved arrows. This causes tensions between the N and I subdomains (zigzag arrow), resulting in their loosening. Bottom, (+) end view of the β subunit’s longitudinal interface in different nucleotide states, as color-coded and shaded by subdomains; to aid viewer’s orientation, the rectangular frame is related to that in panel (A) by 90° rotation; αN moves further toward the (−) end compared with αI, as it has ample space, whereas αI extensively interacts (white shading) with the βN and βC subdomains of the preceding dimer. The GTPase (bolt symbol)-driven transitions broaden this longitudinal interface—as indicated with outlines and white arrows—through additional interactions with αN. Protein structures are shown with SES generated with ChimeraX [71]. The nucleotides are shown as white and heteroatom-colored sticks, and magnesium ion is shown as a ball.