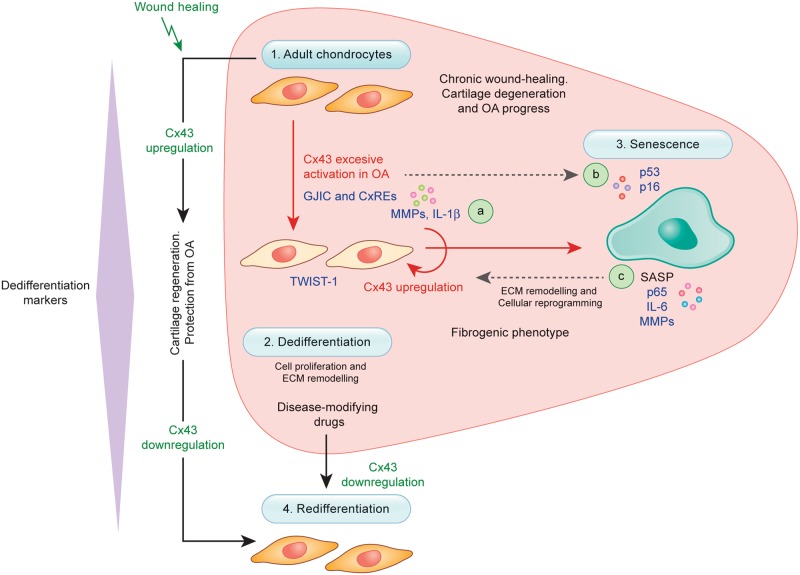
Fig. 5. Downregulation of Cx43 in OA fosters a pro-regenerative environment by favouring redifferentiation.
Accumulation of dedifferentiated (2) and senescent (3) chondrocytes and Cx43 overexpression are typical in the cartilage microenvironment from the early stages of OA. Phenotypic changes associated with upregulation of Cx43 during tissue repair may contribute to ECM remodelling and cartilage regeneration (or wound healing) (1–2 and 4, black arrows). However, they result in a chondrocyte fibrogenic phenotype (2, and red arrow) and accumulation of senescent cells (3) in OA, which is unable to support the ECM composition by continuous activation of ECM remodelling (MMPs) and proinflammatory factors, such as IL-1ß, NF-κB and IL-6 a, c. Downregulation of Cx43 or GJIC (e.g., by CRISPR-Cas9 or CBX treatment) leads to redifferentiation of OACs (4) by reducing Twist-1 activation (2), senescence (3 and b) and SASP (c, NF-κB-p65). The results indicate that dedifferentiation and senescence in OA are flexible processes that can be reverted by modulating Cx43. The elimination or inhibition of factors that contribute to the prolongation of cellular dedifferentiation (Twist-1; chondrocyte-mesenchymal transition) or reprogramming (e.g. via IL-6) reduces the burden of dedifferentiated (2) and senescent (3) cells, which in turn decreases the synthesis of catabolic factors (a and c) involved in cartilage degradation in OA and leads to a mature phenotype able to support the cartilage ECM composition