Figure 1.
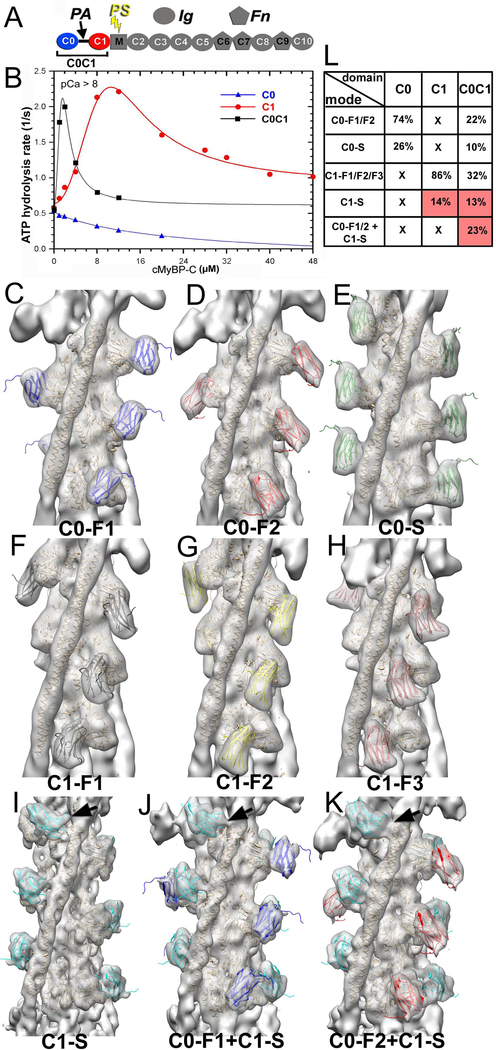
3D reconstructions and corresponding frequencies of structural classes revealed in the C0C1 decorated TFs. (A) cMyBP-C is composed of 8 Immunoglobulin (Ig)-like and 3 fibronectin (Fn)-like domains numbered C0 through C10 starting consecutively from the N-terminus to the C-terminus of the molecule. C0 (blue) and C1 (red) domains are connected by a flexible proline-alanine (PAL) linker, while the M-domain which links C1 and C2 contains functionally significant phosphorylation sites (PS). (B) Effects of C0 (blue curve), C1 (red curve) and tandem C0-C1 (black curve) on TF-dependent myosin-S1 ATPase activity at pCa>8. (C-K) 3D reconstructions of structural classes of C0C1-decorated TFs show all the modes of C0 (C-E) and C1 (F-I) binding to the cTF observed earlier (Harris et al., 2016) along with the two structural classes where C0 and C1 bind simultaneously to the cTF (J and K). High resolution structure of C0 (pdb: 2K1M) is shown in blue (C0-F1 mode), red (C0-F2 mode), or green (C0-S mode). The high resolution structure of the C1 (pdb: 2V6H) is shown in black (C1-F1 mode), yellow (C1-F2 mode), brown (C1-F3 mode), or cyan (C1-S mode). Actin and Tm molecules are shown as tan ribbons. Reconstructions are shown as grey transparent surfaces filtered to the resolution determined for each class (Figure S1C). Interaction of C1 with Tm in the C1-S, C0-F1+C1-S, and C0-F2+C1-S are marked with black arrows. (L) Frequencies for individual classes of cTFs decorated with individual C0 and C1 domains obtained earlier (Harris et al., 2016) are compared with those calculated for the C0C1-decorated TFs. Frequencies of classes where C1 interacts with Tm are marked in red.