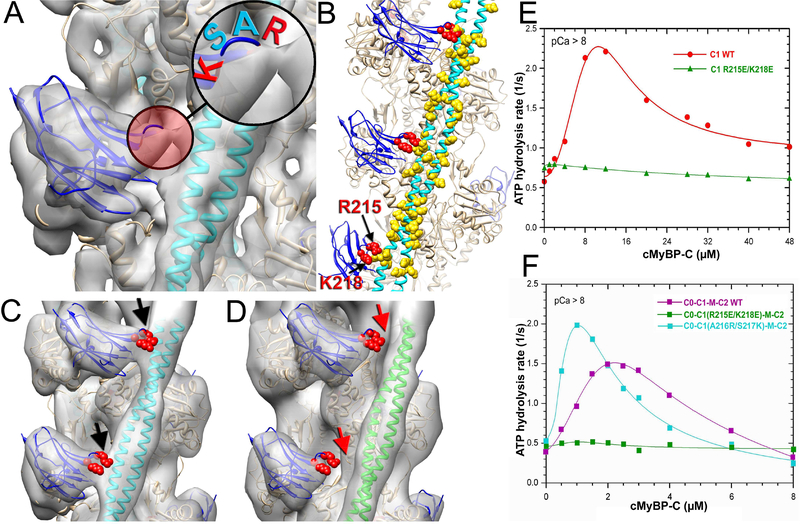
Figure 3.
R215 and K218 amino acid residues of C1 maintain its interaction with Tm which is required for the TF activation by the cMyBP-C NTD. (A) A 9 Å 3D reconstruction of the C1-S class shows a bridge of density (red circle) between C1 (blue ribbons) and Tm (cyan ribbons). Insert shows that C1 loop involved in the interaction is comprised of two positively charged (red) and two neutral (cyan) residues. (B) Residues R215 and K218 (red spheres) of C1 loop are located in proximity to negatively charged residues of Tm (yellow spheres). Tm model is from (Behrmann et al., 2012). (C-D) In the 3D reconstruction of the wild type C1 in the C1-S mode there is a bridge of density (black arrows) between C1 (C, blue ribbons) and Tm in the myosin state (C, cyan ribbons). It is absent in the 3D reconstruction of the C1-S class of the C1(R215E/K218E)-decorated TFs (D, red arrows) where Tm is in the c-closed position (D, green ribbons). (E) Effects of C1 (red curve), and C1(R215E/K218E) (green curve) on TF-dependent myosin-S1 ATPase activity at pCa>8. (F) Effects of C0C1MC2 (magenta curve), C0C1(R215E/K218E)MC2 (green curve), and C0C1(A216R/S217K)MC2 on TF-dependent myosin-S1 ATPase activity at pCa>8.