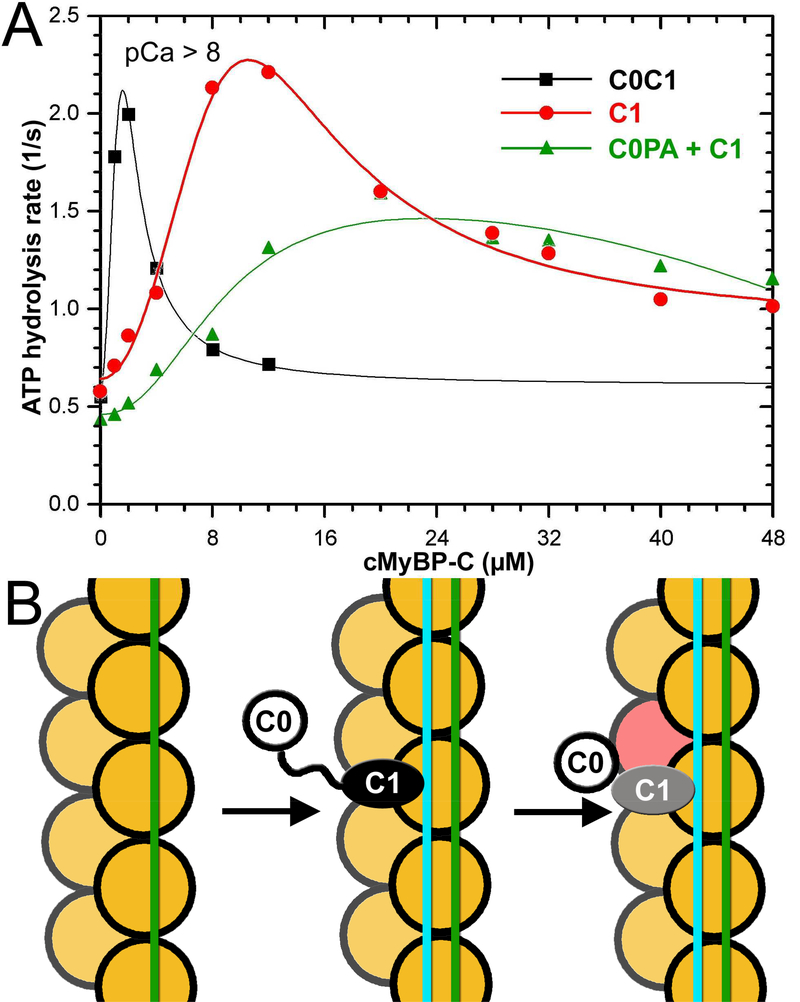
Figure 5.
C0 and C1 Ig-domains of cMyBP-C work in tandem to activate the cTF. (A) Effects of C0-PA-C1 (black curve), C1 (red curve), and equimolar mixture of C0-PA and C1 on TF-dependent myosin-S1 ATPase activity at pCa>8. (B) The model of activation of the cTF by C0-PA-C1 fragment of cMyBP-C. At relaxing conditions Tm is in the inactive (c-closed and c-blocked) states (Risi et al., 2017) (green line). Upon C1 binding in the C1-S mode the Tm cable is azimuthally shifted to its myosin (cyan line) state (Figure 2B) by means of interaction with the R215 and K218 residues of C1 (Figure 3). Bound C1 enforces interaction of C0 with the front of the same actin molecule which enhances the C1 interaction with F-actin (Fig. 4A-C). Actin molecules are shown in yellow. C0 Ig-domain is shown as white circle, C1 domain bound in the C1-S mode is black oval, while when activated by the C0 domain is marked in grey.