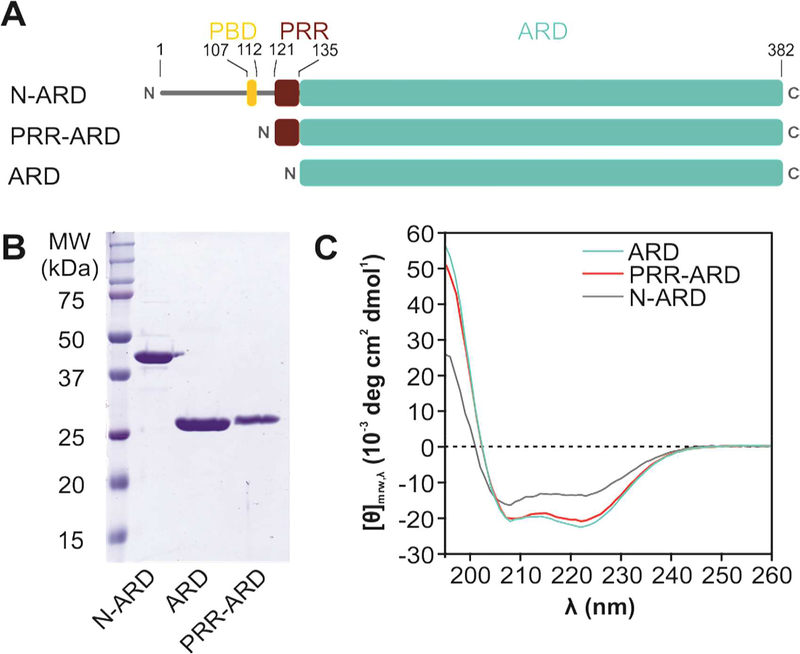
Figure 1: Structural analysis of the TRPV4 N-terminus.
(A) Schematic overview of constructs with varying N-terminus length. The α-helical Ankyrin repeat domain (ARD, cyan) is preceded by a ~130 amino acid stretch harboring the PIP2 binding domain (PBD, yellow) and the proline rich region (PRR, red) important for PACSIN SH3 domain interaction. (B) Coomassie-stained SDS-PAGE of purified chicken TRPV4 N-terminal constructs. (C) CD spectra of the entire N-terminus (N-ARD; residues 1–383), the ankyrin repeat domain with the PRR (PRR-ARD; 122–383), and the isolated Ankyrin repeat domain (ARD; 134–383). (See Fig. S1 for CD studies of human TRPV4 N-terminal constructs).