FIGURE 3.
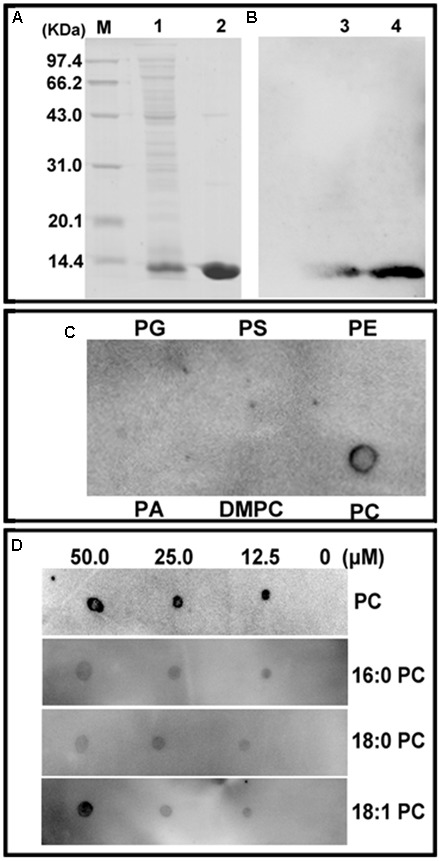
Purification of (His)6-ChACBP1 recombinant protein and its interaction with phosphatidylcholine (PC). (A) Purification of ChACBP1 protein: 9.5-kDa ChACBP1 protein was purified by Ni–NTA agarose and analyzed by SDS-PAGE. M, Marker; Lane 1, pQE30-ChACBP bacterial lysate; lane 2, purified ChACBP1 protein. (B) Western blot analysis of ChACBP1: protein was transferred to nylon membrane, then probed with HRP-conjugated anti-(His)6 antibodies. Lanes 3–4, immunoblot of (His)6-ChACBP1 fusion protein. (C) Binding of (His)6-ChACBP1 and lipid on filter membrane. Lipids (50.0 μM of PG, PS, PE, PA, DMPC, and PC) were spotted onto nitrocellulose and incubated with ChACBP1 protein. Binding of ChACBP1 to lipids was detected by ECL reagent with HRP-conjugated anti-(His)6 antibodies. (D) Binding of (His)6-ChACBP1 to various PC acyl species. Different concentrations (0, 10, 25.0, and 50.0 μM) of 16:0 PC, 18:0 PC, and 18:1 PC were spotted onto nitrocellulose and incubated with ChACBP1. Binding of ChACBP1 to lipids was detected by ECL reagent.
