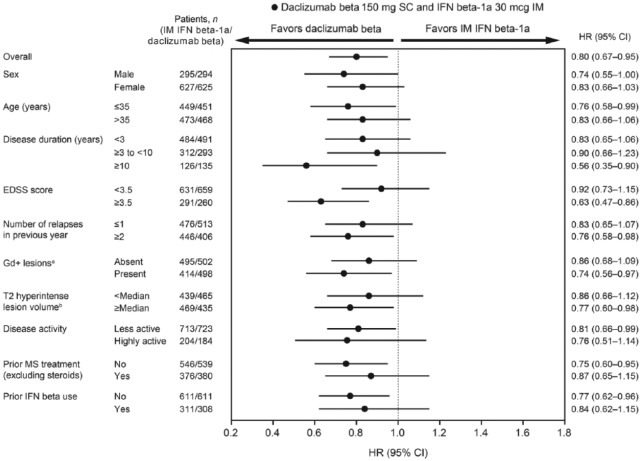
Figure 2.
Forest plot for 24-week sustained modified Multiple Sclerosis Functional Composite progression for daclizumab beta versus IM IFN beta-1a by baseline demographics and disease characteristics.
CI: confidence interval; EDSS: Expanded Disability Status Scale; Gd+: gadolinium-enhancing; IFN: interferon; IM: intramuscular; MS: multiple sclerosis; SC: subcutaneous.
aMissing baseline Gd+ lesions data: IM IFN beta-1a, n = 13; daclizumab beta, n = 19.
bMissing baseline T2 hyperintense lesion volume data: IM IFN beta-1a, n = 14; daclizumab beta, n = 19.
cMissing baseline disease activity data: IM IFN beta-1a, n = 5; daclizumab beta, n = 12.