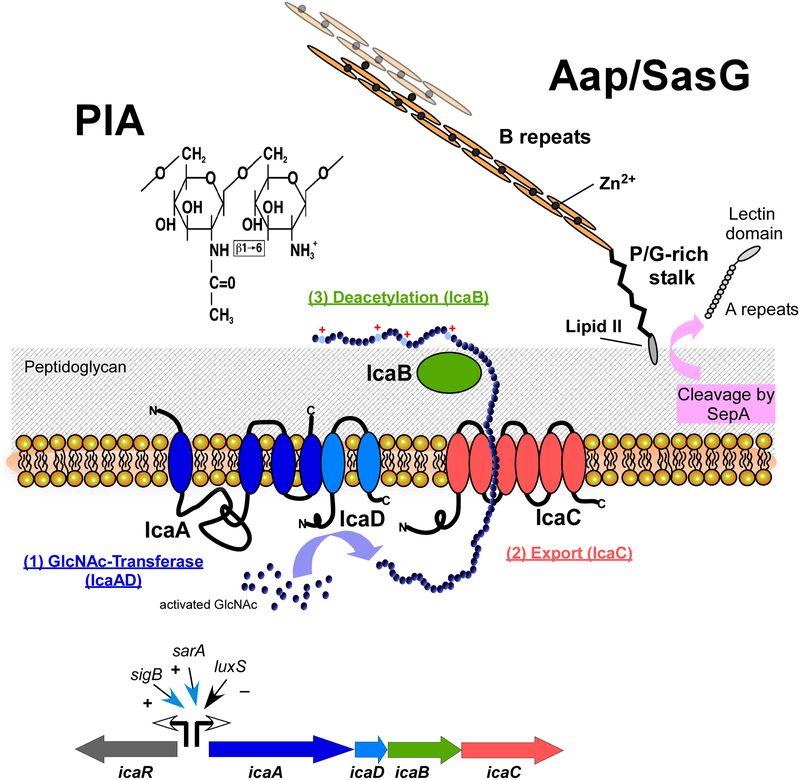
Fig. 2. Predominant staphylococcal biofilm matrix components.
The exopolysaccharide PIA (polysaccharide intercellular adhesin) (left), produced by many S. aureus and S. epidermidis isolates, is a β1–6-linked homopolymer of N-acetylglucosamine. It is synthesized in the cell by the combined activity of the membrane enzymes IcaA and IcaD, and likely exported by IcaC. The extracellular surface-bound enzyme IcaB removes a certain percentage (~ 15 – 20%) of N-acetyl moieties, which gives the otherwise neutral PIA molecule a positive net charge, anchoring PIA to the negatively charged cell surface. In addition to the ica biosynthetic genes icaADBC, the PIA biosynthesis locus also contains a regulatory gene, icaR. Several global regulators impact transcription from the icaR and icaADBC promoters.
The accumulation-associated protein (Aap), which is present in S. epidermidis and has a homologue in S. aureus called SasG, is produced as a 220-kD precursor protein, from which the secreted protease SepA cleaves off the N-terminal A-repeat and lectin domains. It is anchored to the cell wall via sortase-catalyzed covalent linkage to lipid II. Mature Aap forms extended fibrils out of B repeat domains, whose polymerization is dependent on Zn2+ ions. Zn2+ is also required for the interconnection of Aap/SasG proteins from different cells, which can happen in an interspecies manner.