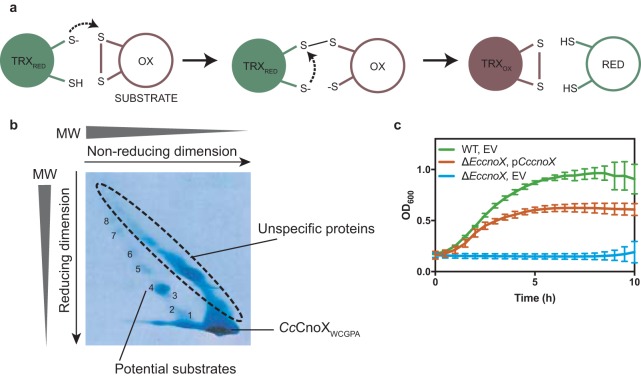
FIG 6.
Ninety proteins depend on the reductase activity of CcCnoX in vivo. (a) Catalytic cycle of Trxs. The first cysteine of the catalytic motif of Trx performs a nucleophilic attack on an oxidized cysteine in its substrate. This leads to the formation of a mixed-disulfide complex between the Trx and its substrate. Then, the second cysteine of the catalytic site of Trx resolves the disulfide, thereby releasing a reduced substrate. (b) Diagonal gel obtained by thiol trapping experiments. The spots analyzed by mass spectrometry are numbered, and potential candidates are identified in Table S1. Here, we show one representative example of this experiment, which has been conducted in triplicate. (c) Growth curves of wild-type cells harboring an empty vector (EV [green]), ΔEccnoX cells plus EV [blue]), and ΔEccnoX cells expressing CcCnoX in trans (orange) show that CcCnoX partially complements an EccnoX deletion under HOCl stress. Cells were grown in M9 + glucose medium after addition of 2 mM HOCl. The OD600 was measured for 10 h. This graph shows the mean from three independent experiments; error bars are standard errors of the mean (SEM).