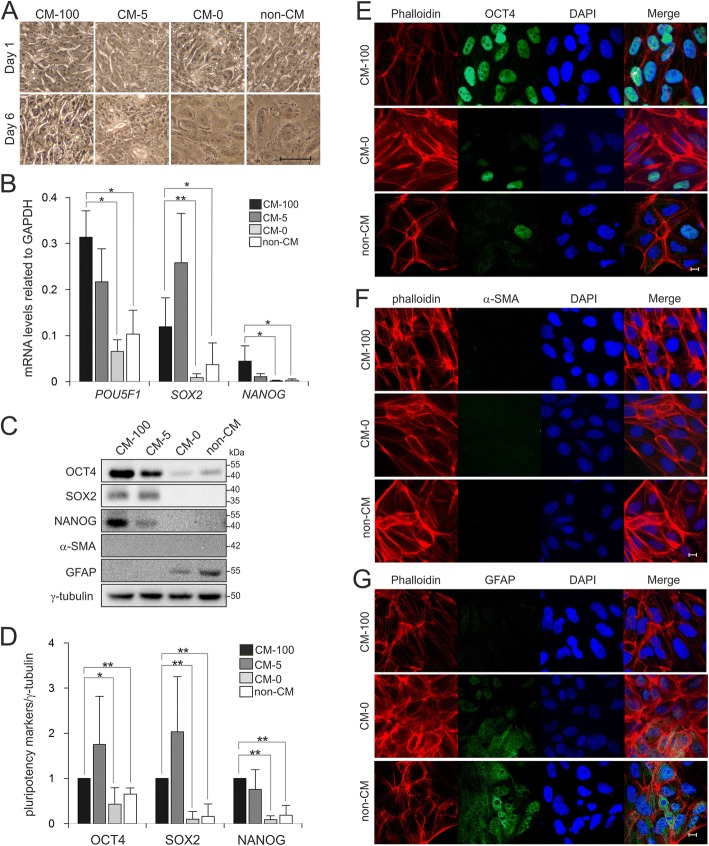
Fig. 2.
The critical role of bFGF for maintaining hiPSC pluripotency. a Phase contrast images of hiPSCs cultured under four different conditions, CM-100, CM-5, CM-0 and non-CM for 6 days. Undifferentiated hiPSCs formed compact colonies (CM-100), while without bFGF supplementation hiPSCs spread and flattened at day 6 (CM-0 and non-CM). Scale bar, 50 μm. b qPCR analysis showed the downregulation of pluripotency markers POU5F1, SOX2 and NANOG in cells cultured in CM-0 and non-CM in comparison to control group (CM-100). All expression values were normalized to GAPDH. Results from three separate experiments, each carried out in triplicate, are shown as mean ± SD (ANOVA; *p < 0.05 and **p < 0.01). c Western blot analysis of pluripotency and differentation markers under four different conditions showed the downregulation of stemness markers and upregulation of GFAP. γ-tubulin served as loading control. d The graph represents densitometric analysis of three independent experiments, each carried out in duplicates. All values were normalized to γ-tubulin and relative to CM-100. Data are shown as mean ± SD (ANOVA; *p < 0.05 and **p < 0.01). hiPSCs were immunostained for OCT4 (e), α-SMA (f) and GFAP (g) under three different culture conditions; CM-100, CM-0 and non-CM. Cell nuclei were stained with DAPI (blue) and F-actin was stained with phalloidin (red). Scale bar, 10 μm