Figure 1.
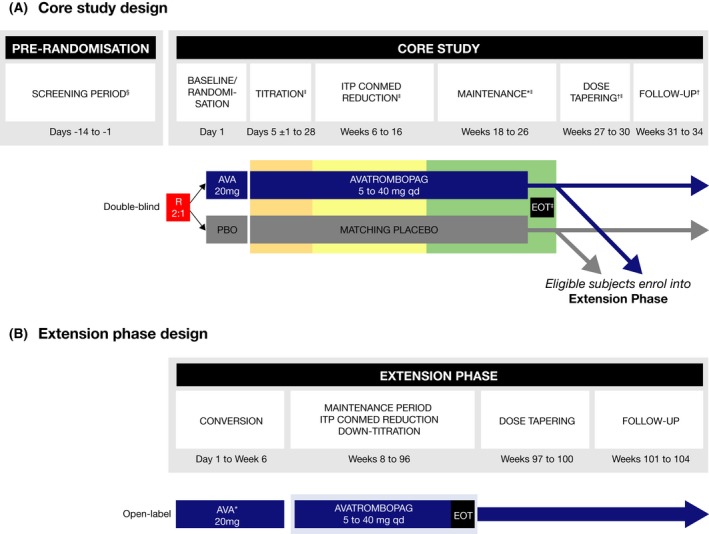
Study design. (A) Core study. (B) Extension phase. AVA, avatrombopag; CONMED, concomitant medication; E, extension; EOT, end‐of‐treatment; ITP, immune thrombocytopenia; PBO, placebo; qd, once‐daily; R, randomised. *At EOT visit (visit 22), patients could enter the extension phase and receive open‐label avatrombopag therapy. Patients who did not continue into the extension phase entered the dose‐tapering and follow‐up phase. †Only for patients who did not enter the extension phase. ‡Optional entry into the open‐label extension phase. §The screening visit and day 1 baseline/randomisation visit platelet counts were averaged to obtain the baseline platelet count value. The two samples were obtained ≥48 h and ≤2 weeks apart and the results were available prior to randomisation. Therefore, an additional screening platelet count may have been required due to issues with scheduling. ‖Patients who discontinued early who met the criteria for a lack of treatment effect may have moved directly into the open‐label extension phase. [Colour figure can be viewed at http://wileyonlinelibrary.com]
