Table 1.
In vitro activity and metabolic stability in liver microsomes of lonaprisan and its human metabolites.
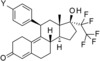
| |||||
|---|---|---|---|---|---|
| Compound | Y | PR transactivation assay | Metabolic stability assay | ||
| IC50 [nm][a] | Efficacy [%] | F max Human [%][a] | F max Rat [%][a] | ||
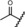
| lonaprisan |
|
0.02 | 100 | 77 | 83 |
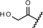
| metabolite 1 |
|
0.14 | 100 | 58 | 81 |
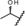
| metabolite 2 |
|
0.10 | 100 | 50 | 57 |
| metabolite 3 |
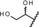
|
3.3 | 100 | 68 | 82 |
[a] Concentration of drug resulting in 50 % inhibition. [b] Maximum oral bioavailability calculated from in vitro hepatic extraction ratio (E H) in liver microsomes, assuming 100 % absorption (F max=1−E H); see the Experimental Section for details.
