Figure 4. Escape from cell cycle arrest is characterized by a sharp switch in the balance between p21 and CDK2 activity.
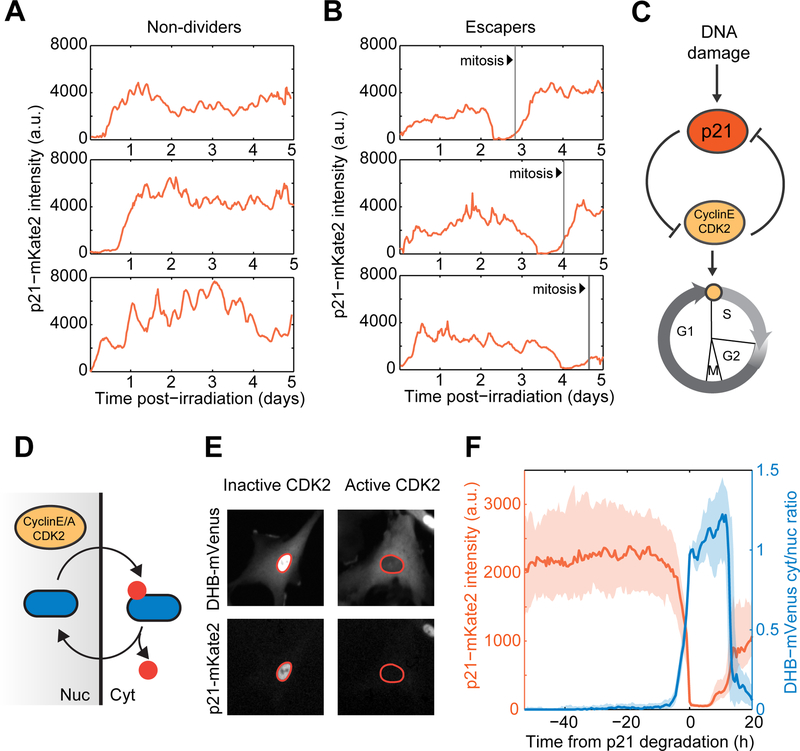
(A,B) Representative single cell trajectories of p21-mKate2 levels in irradiated non-dividers and escapers. Vertical lines denote mitosis events.
(C) p21 arrests the cell cycle by inhibiting the function of the CDK2/CyclinE complex which in turns leads to p21 degradation upon S-phase entry, forming a double negative feedback loop.
(D) CyclinE-CDK2 dependent phosphorylation triggers cytoplasmic retention of DHB-mVenus, which serves as a quantitative proxy for CDK2 activity in live cells.
(E) Representative images of cells harboring CDK2 activity reporter and p21-mKate2 fluorescent protein. Nuclei are outlined in orange.
(F) Dynamics of p21 and CDK2 activity in the vicinity of escape events. Irradiated cells were in silico synchronized to the time of p21 degradation. Bold lines and shaded areas correspond to median and interquartile ranges, respectively (n = 80 cells).
