Figure 6.
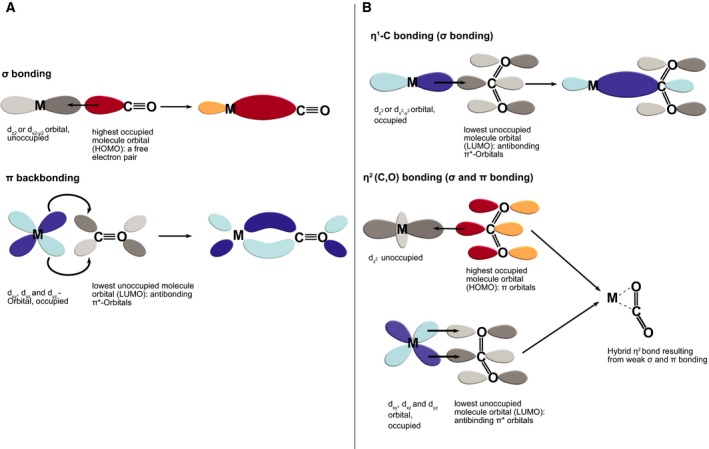
CO (A) and CO 2 (B) bonding to transition metals. (A) CO binds to a transition metal (M) via the free electron pair of its carbon atom. The electron density in this orbital (red = positive phase, yellow = negative phase) can be placed into empty metal d orbitals forming a σ bond. Concurrently, a π bond is formed between an occupied d orbital and the antibonding empty π* orbital of CO (darker grey = positive phase, lighter grey = negative phase), so called ‘π backbonding’. (B) Different bonding modes between CO 2 and transition metals include η1‐C coordination, which mostly happens with electron‐rich metals (i.e. lower oxidation states), as they can transfer charge from the dz 2 orbitals to the antibonding π* orbitals of CO 2. A double bond‐like interaction (dashed line) can also occur between a transition metal, carbon and oxygen, η2‐(C,O) bonding: an empty dz 2 orbital of a metal can take electron density from the π orbital of the CO 2 orbital (red/yellow), while electron density can also be transferred from occupied d orbitals (blue) into the antibonding π* orbitals of CO 2 (comparable to the backbonding of CO, but weaker).
