Figure 2.
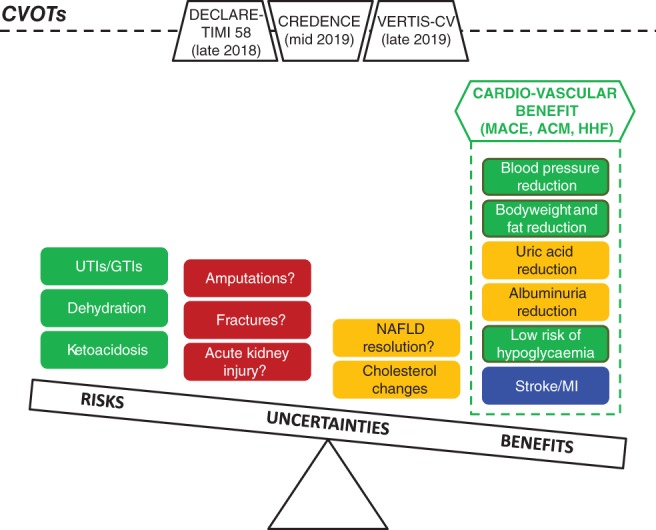
Current evidence on the evolving risk–benefit evaluation of SGLT2‐Is, with ongoing cardiovascular outcomes trials (CVOTs) potentially affecting final assessment. ACM, all‐cause mortality; GTIs, genital tract infections; HHF, hospitalization for heart failure; MACE, major adverse cardiovascular events; MI, myocardial infarction; NAFLD, non‐alcoholic fatty liver disease; UTIs, urinary tract infections. Colour coding: green = CONSOLIDATED EVIDENCE (general agreement among types of evidence†); blue = ENCOURAGING EVIDENCE (partial agreement among types of evidence†); orange = PRELIMINARY EVIDENCE (incomplete/pending data, only from a single type of evidence, namely CVOTs); and red = UNCERTAIN EVIDENCE (conflicting data among types of evidence†). †Including systematic reviews with meta‐analyses, clinical trials, observational and pharmacovigilance studies
