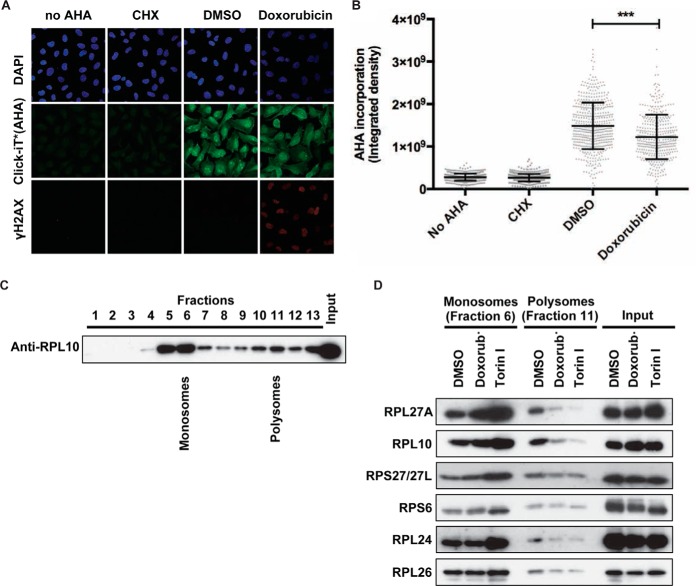
Fig. 4.
DNA damage affects ribosomal function. (A) U2OS cells were synchronized in G2 as described for Fig. 1(A) and treated with doxorubicin for 1 h in methionine-depleted media. After wash out, cells were incubated with l- azidohomoalanine (AHA) for 2 h; cells with no AHA or treated with cycloheximide were used as negative controls. Cells were fixed and incubated with Alexa Fluor 488 alkyne to label AHA incorporation into nascent proteins; cells were counterstained with DAPI to show the nuclei. γH2AX staining is used as a marker for DNA break formation. Panels show representative confocal images from each condition. (B) Scatter plot of individual AHA levels in no AHA, cycloheximide-, DMSO-, and doxorubicin-treated samples from one out of the six experiments. The amount of nascent protein synthesis in each condition was quantified by measuring AHA fluorescence intensity per cell using a macro developed for this purpose. Each bar represents mean ± S.D. from each condition. Statistical significance was determined using nonparametric Kruskal-Wallis test (***p < 0.0001). (C) Lysates from DMSO-treated U2OS cells were fractionated in sucrose density gradients to isolate the monosomes and polysomes. Thirteen fractions and the input were separated by SDS-PAGE and immunoblotted with anti-RPL10 antibody. Fractions 6 and 11 were assigned as representative monosome- and polysome-enriched fractions. (D) Lysates from cells treated with DMSO, doxorubicin, and Torin I were fractionated in sucrose density gradients to isolate the monosomes and polysomes. Fractions 6 and 11 from each sample were separated by SDS-PAGE and immunoblotted with indicated antibodies.