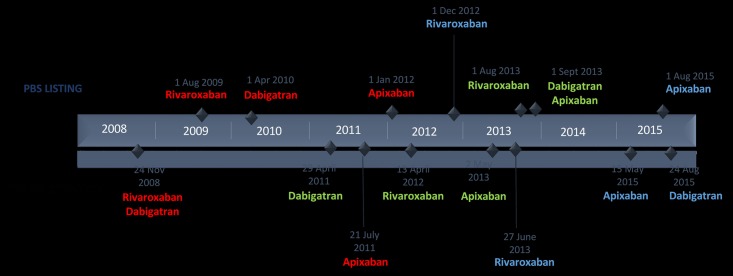
Fig 1. Timeline of NOAC market entry in Australia.
Notes: The dates shown above the timeline are the PBS listing (reimbursement) dates while the dates shown below the timeline are the Therapeutic Goods Administration (TGA) registration (market approval) dates for each of the approved indications for NOAC use. Legend: Red: prevention of venous thromboembolic events (VTE) in adult patients who have undergone elective total hip or total knee replacement surgery; Green: prevention of stroke and systemic embolism in (adult) patients with non-valvular atrial fibrillation (AF) and at least one additional risk factor for stroke; Blue: Treatment (Tx) of deep vein thrombosis (DVT) and pulmonary embolism (PE) and for the prevention (Pv) of recurrent DVT and PE in adults.