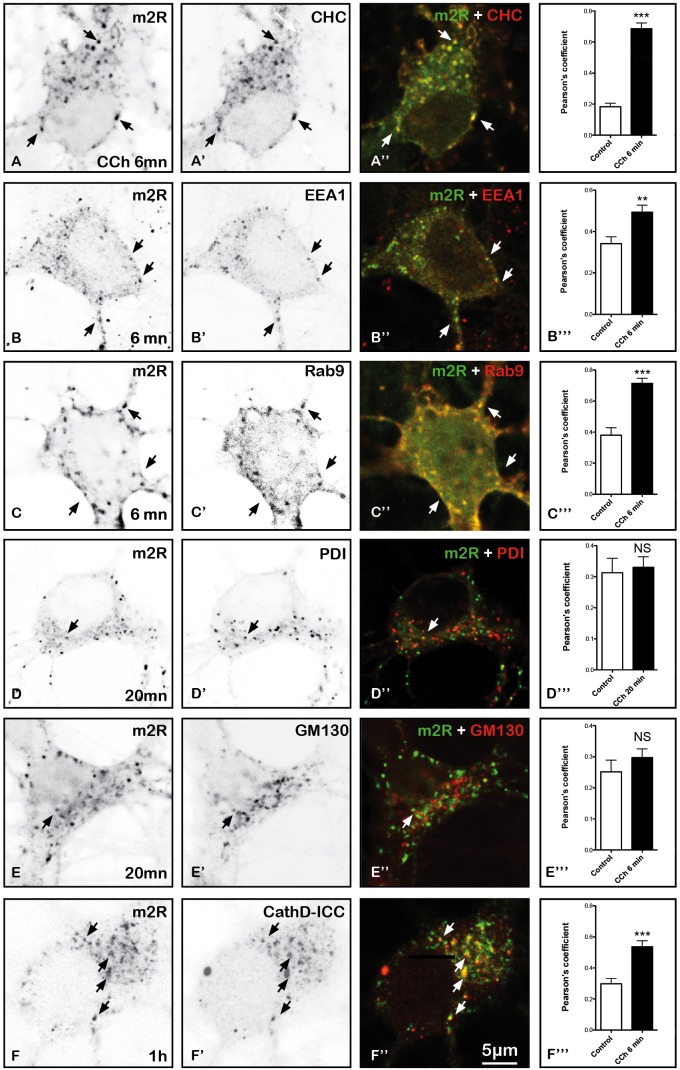
FIGURE 9.
Immunohistochemical localization of m2R in neuronal compartments involved in endocytosis, synthesis, maturation, and degradation in fixed hippocampal neurons. Hippocampal neurons were transfected with m2R-WT. Neurons were stimulated with CCh at 30 μM for 6, 20 min and 1 h fixed, and processed for visualization of m2R together with markers of intraneuronal compartments and observed by confocal microscopy. (A–C”) 6 min after CCh stimulation (30 μM), some m2R immunopositive punta colocalize with CHC in clathrin-coated pits, EEA1 in early endosomes and Rab9 in late endosomes (arrow heads). (D–E”) Twenty minutes after CCh stimulation (30 μM), we failed to detect no colocalization of m2R with PDI, a marker of endoplasmic reticulum and GM130, a marker of Golgi apparatus. (F–F”) One hour after CCh stimulation (30 μM), some m2R immunopositive puncta colocalize with CathD, a marker of lysosomes (arrow heads). The quantitative analysis of the colocalization of m2R and markers of subcellular compartment in neurons was performed using the Jacop Plugin of ImageJ and statistical data are reported from the Costes’s randomization-based colocalization module (see Materials and Methods). Data are expressed as a Pearson’s coefficient (pc) and pc were compared using the Mann–Whitney U-test. Our analysis shows that the colocalization of the immunofluorescent signals for m2R with CHC, EEA1, Rab9, and CathD is higher after treatment with CCh compared to untreated neurons (CHC, Rab9, and CathD: ∗∗∗p < 0.0001; EEA1: ∗∗p < 0.01). In contrast, the colocalization of the immunofluorescent signals for m2R with PDI and GM130 do not significantly differ in CCh-treated neurons compared to untreated Control neurons: CHC n = 20, EEA1 n = 16, Rab9 n = 15, PDI n = 21, GM130 n = 17, CathD n = 17; CCh-treated neurons : CHC n = 12, EEA1 n = 18, Rab9 n = 15, PDI n = 20, GM130 n = 18, CathD n = 15.