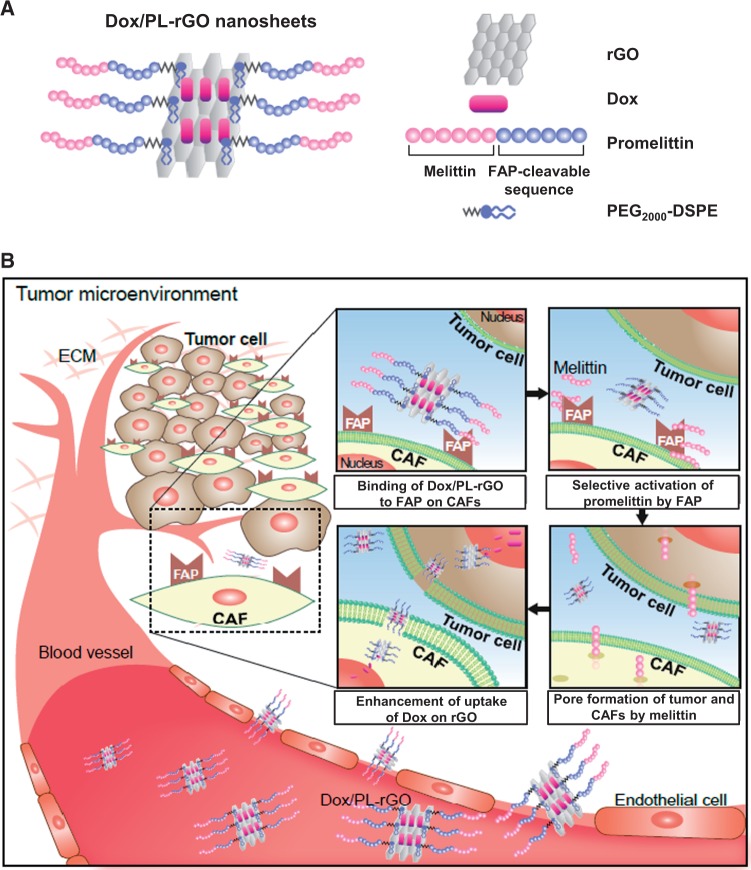
Figure 1.
Nanostructure of doxorubicin-loaded promelittin lipid derivative–reduced graphene oxide (Dox/PL-rGO) nanosheets and a schematic depiction of the hypothesized mechanism underlying their action. A) The promelittin lipid derivative, PL, containing a fibroblast activation protein (FAP)–cleavable sequence, was anchored onto rGO nanosheets. The resulting PL-rGO was further loaded with Dox, yielding Dox/PL-rGO. B) Schematic depiction of the presumed mechanism of Dox/PL-rGO. Activation of the promelittin moiety of PL-rGO by FAP overexpressed on cancer-associated fibroblasts releases melittin. Diffusion of melittin to surrounding tumor cells and cells in the tumor microenvironment promotes formation of pores in the membrane, increasing the cellular uptake of Dox-loaded rGO nanosheets and enhancing anticancer efficacy. CAFs = cancer-associated fibroblasts; Dox = doxorubicin; FAP = fibroblast activation protein; PL = promelittin lipid derivative; rGO = reduced graphene oxide.