Table 3.
Effect of ring structure on the calculated 1 e− potentials for oxidation and reduction of cyclic aminoxyl radicals. Gas phase species were optimized at the G3(MP2)-RAD level with B3-LYP/6–31G(d) level of theory. Solvation energies of each species were calculated using the polarized continuum model. Experimental values were measured in CH3CN with 0.1 M NBu4BF4 at a Pt electrode. Values are referenced against NHE.78
| Entry | Aminoxyl Radical |
Experimental Potential (mV) | Calculated Potential (mV) |
|---|---|---|---|
| 1 | 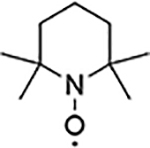 |
850 | 807 |
| 2 | 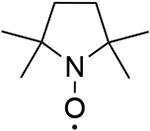 |
976 | 971 |
| 3 | 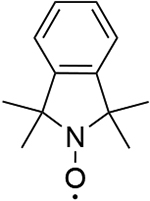 |
1045 | 999 |
| 4 | 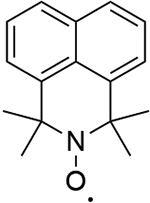 |
1010 | 474 |
