Table 5.
Experimental N-hydroxyimide/imidoxyl redox potentials vs. NHE.a
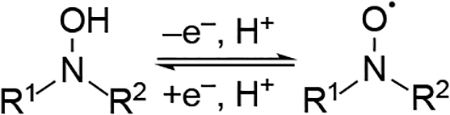
| Entry | N-Hydroxyimide Derivative | E1/2a | Ref. | Entry |
N-Hydroxyimide Derivative |
E1/2a | Ref. |
|---|---|---|---|---|---|---|---|
| 1 |  |
0.94b | 246 | 9 | 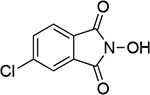 |
||
| 0.93c | 247 | 0.95b | 246 | ||||
| 1.08d | 249 | ||||||
| 2 |  |
10 |  |
||||
| 1.16c | 247 | 0.98b | 246 | ||||
| 3 | 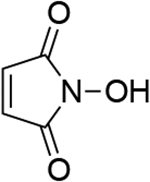 |
11 | 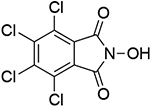 |
||||
| 1.01c | 247 | 1.00b | 246 | ||||
| 4 | 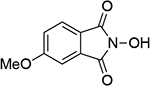 |
12 | 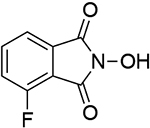 |
||||
| 0.91b | 246 | ||||||
| 1.04d | 249 | 1.12b | 249 | ||||
| 5 | 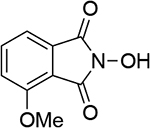 |
13 |  |
||||
| 1.05d | 249 | ||||||
| 1.03b | 246 | ||||||
| 6 | 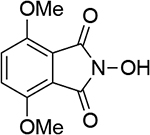 |
14 | 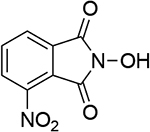 |
||||
| 1.04d | 249 | 1.02b | 246 | ||||
| 7 | 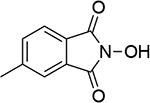 |
15 | 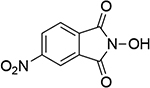 |
||||
| 1.06d | 249 | 0.97b | 246 | ||||
| 8 | 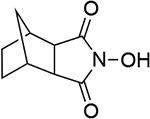 |
16 | 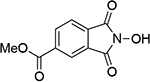 |
||||
| 0.90b | 246 | ||||||
| 1.19d | 250 | 1.13d | 249 | ||||
Experimental measurements performed with 1.7 equivalents 2,4,6-collidine in CH3CN.
Experimental measurements performed with 2 equiv 2,4,6-collidine in CH3CN.
Experimental measurements performed in 50 mM citrate buffer (pH 5).
Experimental measurements performed 45 mM phosphate buffer (pH 6).250
