Figure 3.
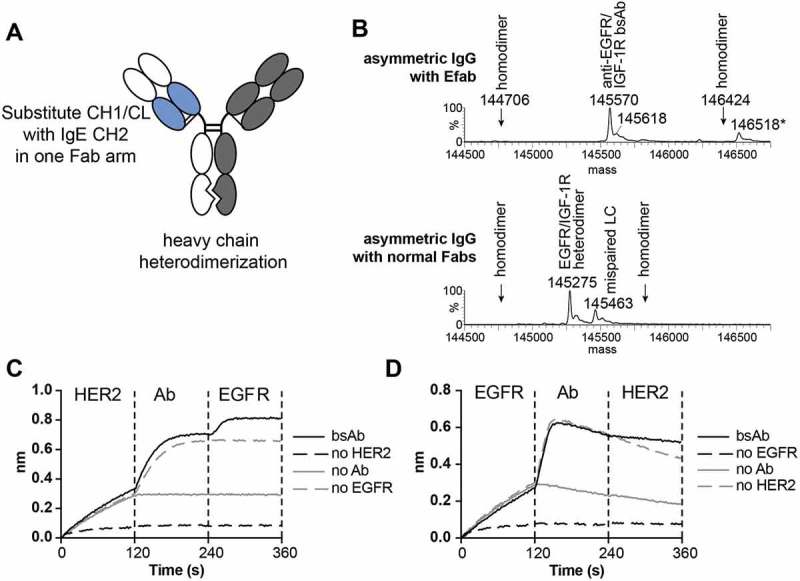
A) Cartoon of EFab-containing asymmetric IgG bispecific antibody. EFab domain substitutions are colored in blue. Heterodimerization mutations are present in CH3 domain. B) Deconvoluted mass spectra of asymmetric IgGs with and without EFab. Anti-EGFR M60-A02 and anti-IGF1R C06 make up the antibody pair, with the EFab on the anti-EGFR arm. The correctly formed EFab containing bispecific has an expected mass of 145,567 Da (145,570 Da observed; the peak labeled * is the correctly formed bispecific antibody plus an O-glycan). There is no observed mispairing of light chain for the EFab molecule, (+ 312 Da for two IGF1R light chains and – 312 Da for two EGFR light chains), but significant mispairing of the regular Fab-containing construct is observed, resulting in antibody containing two M60-A02 light chains. C) and D) Binding by BLI of bispecific antibody to HER2 and EGFR (trastuzumab/cetuximab) in a sandwich format in both directions. Anti-penta-His biosensors were loaded with His-tagged antigens at 5 µg/ml, followed by bispecific antibodies (20 nM) and second antigen (15 µg/ml).
