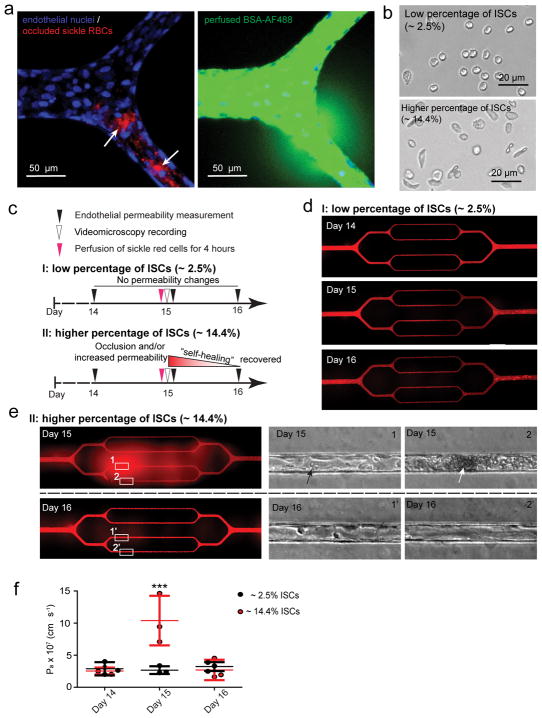
Figure 4. Sickle red cell-endothelial cell interactions in and of themselves induce microchannel occlusion and loss of endothelial barrier function in the engineered microvasculature.
a) Representative 3D confocal microscope images of endothelialized microchannels occluded by sickle RBCs (pre-stained with R18; pointed by arrows) and the resultant, co-localized increased endothelial permeability and leakage of BSA-AF488 in situ. b) Representative bright field images of blood smears reveal two experimental scenarios involving our SCD patients’ blood samples. c) The experimental timeline and design. d) Representative stitched composite of epi-fluorescence images indicate that perfusion of low percentages of ISCs for 4 hours caused no change in permeability in the engineered microvasculature. e) Representative stitched composite of epi-fluorescence images and brightfield microscopy images of the boxed regions indicate that perfusion of high percentages of ISCs for 4 hours caused temporary microchannel occlusion and was sufficient, in and of itself and in the absence of other blood cell populations or exogenous inflammatory mediators, to induce loss of endothelial barrier function and increased permeability in the engineered microvasculature. Within 1 day of continued culture and perfusion, the ISC microchannel occlusion cleared and the engineered microvasculature “self-healed”, gradually recovering its barrier function (black arrow: adherent sickle RBCs; white arrow: RBC aggregate that occludes the microchannel). f) Quantitative apparent permeability measurements of the BSA tracer at different time points of both pre- and post-perfusion of ISCs from sickle cell patients. The data was plotted as the mean ± s.d. with n=3 independent replicates. Samples with either low or high ratio of ISC were obtained from three different SCD patients. P-values were calculated with two-way ANOVA with Bonferroni’s post hoc test (*** P<0.001).