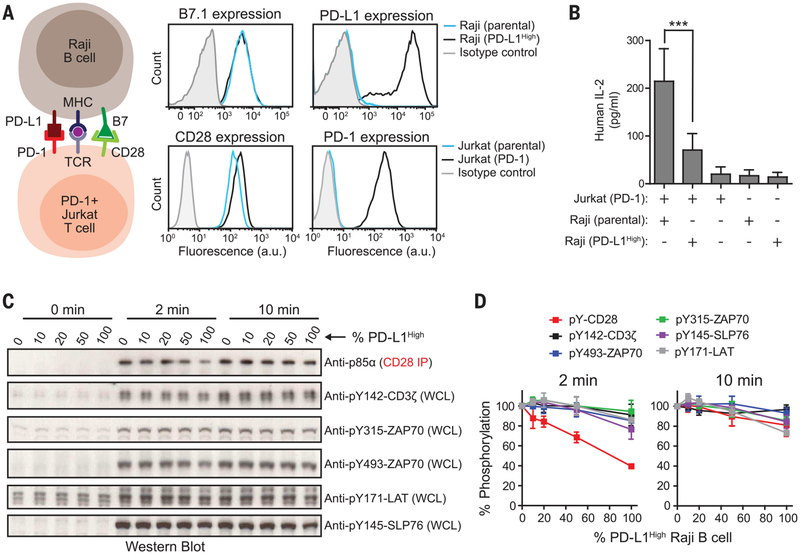
Fig. 4. Intact cell assays confirm CD28 as the preferential target of PD-1–mediated inhibition.
(A) The cartoon on the left illustrates an intact cell assay in which CD28+, PD-1–transduced Jurkat T cells were stimulated with B7.1+, PD-L1–transduced (PD-L1High) Raji B cells pre-loaded with antigen. On the right are FACS (fluorescence-activated cell sorting) histograms showing the expression of B7.1 and PD-L1 in parental or PD-L1High Raji B cells and the expression of CD28 and PD-1 in parental or PD-1–transduced Jurkat T cells. a.u., arbitrary units. (B) Bar graph summarizing IL-2 release from a 24-hour Jurkat-Raji coculture with or without PDL1–PD-1 signaling and from each type of cell alone (see the methods). Data are presented as means ± SD from four independent measurements, with each run in triplicates. ***P < 0.0001; two-way ANOVA (analysis of variance). (C) A representative Western blot experiment showing the phosphorylation of CD28 and TCR signaling components in Jurkat Tcells in response to PD-L1 titration on antigen-presenting Raji B cells; the time after the initial contact of the two cell populations is indicated (see the methods). Different ratios of PD-L1High to PD-L1– Raji B cells (both containing pMHC and B7.1) were used to vary the PD-L1 stimulation to the Jurkat cells. Each condition con tained an identical number of Raji B cells (Raji to Jurkat ratio, 0.75). The phosphorylation states of CD3ζ, ZAP70, and LAT were immunoblotted with phosphospecific antibodies. Because of the lack of CD28-specific phosphotyrosine antibodies, CD28 was coprecipitated with p85α (see the methods), which is dependent on CD28 phosphorylation. WCL, whole cell lysate. (D) Quantification of phosphorylation data, incorporating results from three independent experiments (means ± SD).