Figure 1:
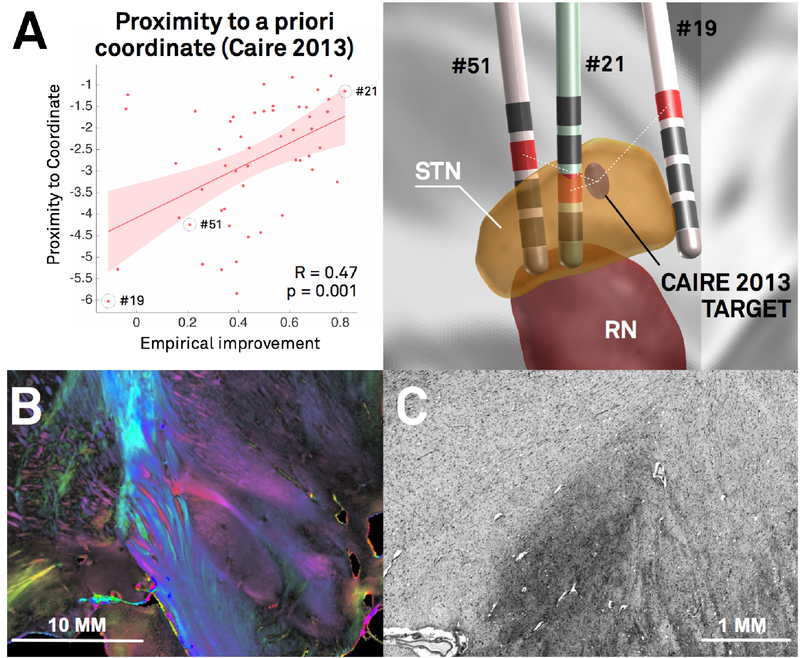
In DBS imaging, “millimeters matter”, which poses specific methodological challenges. A) Re-analysis of the Berlin cohort described in (Horn et al., 2017c) shows that proximity of active contact centers to an optimal target is predictive of %-UPDRS-III improvement (left). The target was defined in a meta-analysis (Caire et al., 2013) and transformed to MNI space in a probabilistic fashion (Horn et al., 2017a). Active contacts between electrodes of patients 51 and 21 are a mere two mm away from each other, but result in largely different clinical results (right). The same distance (two mm) corresponds to the average image resolution of functional MRI for which many neuroimaging tools were initially developed. Thus, in the field of DBS imaging, the distance of two mm plays a crucial role, whereas it is often considered insignificant in common neuroimaging studies. B) Coronal polarized light imaging section of the human subthalamic nucleus with surrounding tracts. Image courtesy by Prof. Karl Zilles and Dr. Markus Axer, Forschungszentrum Jülich, INM-1. C) Coronal section of the BigBrain dataset (Amunts et al., 2013) as visualized in the microdraw online application (http://microdraw.pasteur.fr/). Cell sparser and denser subregions are discernible, potentially corresponding to functional zones of the nucleus (Marani et al., 2008). B) and C) demonstrate the tightly-packed anatomical complexity of the STN-DBS target region that is similarly reflected in clinical outcome (A). Of note, only a subregion of this small nucleus is considered an optimal DBS target. The combination of such small and complex DBS targets with a potentially huge impact of small misplacements poses extreme challenges to the field of DBS imaging and raise the need for high-precision pipelines.