Abstract
Background:
Cabozantinib can enhance the effect of abiraterone in preclinical prostate cancer models. This study aimed to define the recommended phase 2 dose (RP2D) and preliminary efficacy of abiraterone+cabozantinib in mCRPC.
Methods:
Patients with progressive mCRPC with 0–2 prior chemotherapy regimens but no prior CYP17A1 or MET inhibitor received abiraterone acetate at 1000mg daily with prednisone 5mg BID in combination with cabozantinib at 20mg, 40mg, or 60mg daily in a dose-escalation 3+3 open-label phase 1 design (Part A). After tolerable doses were defined, cohorts were expanded to better define toxicity and efficacy (Part B).
Results:
There were no dose-limiting toxicities (DLTs) in the first 4 weeks at any of the 3 dose levels in Part A. Two of the 3 patients at the 60mg dose level required dose reductions beyond cycle 2 due to fatigue. In Part B, 9 more patients were accrued to each of the 20mg and 40mg doses. Of the 12 patients treated at the 40mg dose, only one DLT (grade 3 lipase elevation) was observed in cycle 1. The median time to radiographic progression was 12.88 months (95% CI:5.42- not estimated [NE]) in the 20mg cohort and 22.01 months (95% CI:15.44-NE) in the 40mg cohort. Median overall survival was 23.29 months (95% CI:19.06-NE) in the 20mg cohort and 39.08 months (95% CI:17.38-NE) in the 40mg cohort.
Conclusions:
Based on tolerability and preliminary efficacy, 40mg cabozantinib plus 1000mg abiraterone daily is the RP2D.
Keywords: prostate cancer, abiraterone, cabozantinib, castration-resistant, XL184
Introduction
While most men diagnosed with prostate cancer do not die of their disease, prostate cancer is the third most common cause of cancer death among men in the United States1. The initial systemic treatment for metastatic prostate cancer is androgen deprivation therapy (ADT), but eventually prostate cancer cells become resistant to this intervention and transition to a state of castration resistance2. In a significant number of patients, castration-resistant prostate cancers remain dependent on signaling through the androgen receptor (AR)3–5. Abiraterone acetate is an inhibitor of 17α-hydroxylase/C17, 20-lyase (CYP17A1) that blocks the synthesis of androgens by the adrenal glands6 and prostate cancer cells themselves7, thus depriving these cells of non-testicular sources of androgens. Abiraterone is approved for the treatment of mCRPC both in patients who have been previously treated with docetaxel chemotherapy8, and in patients who are chemotherapy naïve9. More recently, abiraterone has been reported to prolong overall survival in patients with treatment-naïve metastatic hormone sensitive prostate cancer (mHSPC) when combined with initial ADT.10,11 Thus, therapeutic strategies to enhance and prolong the anti-cancer activity of abiraterone are critical to optimize outcomes in patients with metastatic prostate cancer. A variety of mechanisms of resistance to abiraterone have been described in the literature12, including maintaining hormonal signaling through amplification, mutations, and/or splice variants of the AR, or overexpression of CYP17A17; transdifferentiation to a non-AR dependent state13 (including neuroendocrine prostate cancer); and activation of alternate survival signaling pathways.
Cabozantinib is a multi-kinase inhibitor with activity against MET and VEGFR214, as well as multiple other kinases including RET, KIT, FLT-1/3/4, TIE2, and AXL15. Single agent cabozantinib has demonstrated meaningful clinical activity in metastatic CRPC, with results of a Phase II study demonstrating improvements in bone scans, pain, and even measurable soft tissue disease in some patients16. In the randomized Phase III COMET-1 trial17, cabozantinib did not improve overall survival compared with prednisone, however, it did improve bone scan response and progression-free survival.
As such, cabozantinib has shown some activity in very late stage mCRPC but further development would require identification of predictive biomarkers or rational therapeutic combinations likely earlier in the disease process. One such potential therapeutic combination is cabozantinib with abiraterone. In pre-clinical models of CRPC, abiraterone treatment led to a compensatory increase in phosphorylation of the insulin-like growth factor I receptor (IGFIR) pathway with downstream activation of MEK1/2 and ERK1/218, suggesting this pathway as an alternate signaling pathway conferring resistance to abiraterone. In vivo, cabozantinib inhibited abiraterone induction of IGFIR and downstream activation of p-MEK1/2 and p-ERK1/2 and enhanced the anti-tumor activity of abiraterone.18 These studies would suggest that cabozantinib plus abiraterone could be an effective therapeutic strategy for mCRPC or metastatic hormone sensitive prostate cancer. Thus, we conducted a Phase I study to define the recommended phase 2 dose (RP2D) or MTD of cabozantinib when combined with abiraterone, as well as to assess the toxicity and preliminary anti-tumor activity of abiraterone in combination with cabozantinib in mCRPC.
Patients and Methods
Study design and treatments
This two-stage study consisted of a dose-escalation stage (Part A) followed by a dose-expansion stage (Part B). Part A employed a standard 3 + 3 design to evaluate three dose levels of cabozantinib (20, 40 and 60mg/day orally) in combination with abiraterone acetate 1000mg orally daily and prednisone 5mg orally twice daily to establish the recommended phase 2 doses (RP2D) of the combination. Part B was an expansion phase including up to 3 dose levels determined to be safe and tolerable in Part A. The cohorts could be expanded to a maximum of 12 subjects at each dose level (including the subjects from Part A). Treatment in all cohorts was continued until disease progression, unacceptable toxicity, initiation of another cytotoxic or investigational agent, or discontinuation at the investigator’s discretion.
Study population
The study enrolled patients with mCRPC with symptomatic or radiographic progression from prior therapy. Patients may have received 0–2 prior chemotherapy regimens for CRPC; thus, patients could be docetaxel-naïve or refractory. Patients who received prior Met or VEGFR inhibitors were excluded. While treatment with prior CYP17A1 inhibitors was exclusionary, prior ketoconazole was allowed if >120 days elapsed since discontinuation. Continuation of androgen deprivation therapy with a luteinizing hormone-releasing hormone agonist or antagonist was required in patients who were not surgically castrate. Patients were required to have an Eastern Cooperative Oncology Performance Status (ECOG PS) of 2 or less, and demonstrate no major organ dysfunction or uncontrolled hypertension (defined as systolic blood pressure greater than 140 mmHg, or diastolic blood pressure greater than 90 mmHg).
The study was conducted at a single institution in the United States. The study protocol (Dana-Farber/Harvard Cancer Center protocol # 11–441) and its amendments were approved by the Institutional Review Board and all patients provided written informed consent prior to participating in the study. The study was conducted under the principles of the World Medical Association, Declaration of Helsinki, and Good Clinical Practice guidelines of the International Conference on Harmonization.
Pharmacokinetic studies
Samples to determine steady state trough concentrations (Cminss) of abiraterone and cabozantinib in plasma were collected shortly before dosing on days 8, 15, and 21 of cycle 1 and day 1 of cycle 2. Patients were instructed to take both drugs at the same time every day, at least 2 h after food consumption, and at a time that would allow them to arrive at the outpatient clinic for collecting a blood sample before dosing. They were also asked to maintain a diary to record the date and time that each daily dose was taken. At each time point, peripheral venous blood was collected into two tubes containing potassium ethylenediaminetetraacetic acid which were promptly centrifuged (1,300 g, 15 min, 4°C). The plasma was stored in polypropylene cryovials at −80°C until packaged on dry ice for shipment to the analytical laboratories.
Abiraterone was measured by the Clinical Pharmacology Core of the Dana-Farber/Harvard Cancer Center (Boston, MA) using a liquid chromatography/mass spectrometry assay as previously reported with minor modifications, including the use of abiraterone methyl ether as an internal standard.19 The analytical method was validated and applied to the analysis of study samples as recommended in the applicable FDA Guidance for Industry.20 Abiraterone was determined at concentrations ranging from 0.25–100 ng/mL with an interday accuracy of 96.1–105.3% and precision ranging from 2.9–11.2%. The determination of cabozantinib was performed under the direction of Exelixis, Inc. (San Francisco, CA) using a validated liquid chromatography/tandem mass spectrometry assay with a 0.5 ng/mL lower limit of detection.21
Prior clinical pharmacokinetic studies found that steady state pharmacokinetics for repeated daily dosing was achieved within 8 days for abiraterone (mean terminal half-life, 10.3 h) and 15 days for cabozantinib (mean terminal half-life, 111–131 h).22–24 Mean Cminss values were calculated for each patient as the geometric mean of the evaluable results for all four time points for abiraterone and the three time points beginning with day 15 of cycle 1 for cabozantinib. The actual time that samples were collected relative to prior dose had to be known and within 24 ± 4 h of the prior dose for an assay result to be evaluable. Samples collected after the dose of either drug was reduced were inevaluable. The geometric mean Cminss was calculated from the values for individual patients at each dose level and reported with the SD as estimated by the jacknife technique.25 Routines provided in Microsoft Office Excel 2007 (12.0.6715.5000) SP3 MSO (12.0.6683.5000) (Redmond, WA) were used for descriptive statistics.
Safety and antitumor assessments
Safety
Patients were evaluated weekly for the first cycle (DLT assessment period), then every 2 weeks in the second cycle, and then every 28 days until study drug discontinuation. Safety and tolerability were determined by assessment of adverse events (AE), physical examinations, ECOG PS, vital signs, and laboratory tests. The severity of abnormal laboratory values and AEs were classified using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. For assessment of dose-limiting toxicities (DLTs), patients must have completed at least 28 days of therapy and be assessable for toxicity, i.e. any patient who stopped therapy prior to receiving 85% of planned dose in the first four weeks for any reason other than a DLT was required to be replaced.
Antitumor Effect – Serologic Response
PSA responses are reported as the percentage change from the time of their first treatment with therapy. PSA levels were assessed before study entry then every 4 weeks thereafter. The maximum decline in PSA that occurred at any point after treatment is reported using a waterfall plot. PSA progression was the date that a 25% or greater increase and an absolute increase of 2 ng/mL or more from the nadir was documented, which was confirmed by a second value obtained 3 or more weeks later.
Antitumor Effect – Radiographic Response
As a secondary endpoint, measurable and/or non-measurable disease were assessed by RECIST 1.1 criteria using 99mTc-MDP skeletal scintigraphy and contrast-enhanced diagnostic CT of the abdomen and pelvis. Imaging studies were obtained at baseline, and participants were initially reevaluated every 8 weeks (within 7 days prior to day 1 of the subsequent cycle). After the completion of 12 cycles of therapy, participants underwent radiographic evaluation every 12 weeks (within 7 days prior to day 1 of the subsequent cycle). Confirmatory scans were obtained at least 4 weeks following initial documentation of an objective response. Radiographic progression as defined by RECIST 1.1 and Prostate Cancer Clinical Trials Working Group 2 (PCWG2) criteria26 and response assessments were prospectively performed by independent radiologist in the DF/HCC imaging core.
Analysis Methods
All disease outcomes or response assessments were performed on an intent-to-treat (ITT) population, i.e., all patients received response assessments to the initial assigned dose levels. Patient characteristics were summarized descriptively. Discrete variables were summarized as number and percent (%) while continuous variables were summarized by median (interquartile range [IQR]). The safety population included all patients who had taken at least one dose of abiraterone and cabozantinib. The frequency and percent of toxicities measured by the maximum grade a patient experienced is summarized overall and according to cabozantinib dose levels.
The sample size of 12 patients for each dose level in expansion cohort was based on constructing a 2-stage exact binomial confidence interval by the method of Atkinson and Brown27 on the rate of DLTs: if 2 of 12 patients have a DLT (either 0 of 3 in Part A and then 2 of 9 in the expansion cohort , or 1 of 6 in Part A and then 1 of 6 in the expansion cohort), this corresponds to a 16.7% observed DLT rate, and has a 90% confidence interval of either (0.05, 0.63) or (.06, .58).
Per the original protocol, patients were discontinued from study for progressive disease due to radiographic progression by PCWG2 criteria,26 or in the absence of radiographic progression if there was symptomatic progression or changes in the participant’s condition rendering the participant unacceptable for further treatment in the opinion of the treating investigator. This latter group of patients were defined on retrospective analysis as having progressed due to “no longer clinically benefiting” (NLCB) as suggested by PCWG3 criteria,28 which were published after this study was conducted. Time to event outcomes, such as (i) time to PSA progression, (ii) time to radiographic progression by PCWG2 criteria26 per original protocol, (iii) time to progression as defined as the earlier of radiographic progression (PCWG2)26 or clinician assessment of NLCB28 and (iv) overall survival (OS) were estimated using the Kaplan-Meier method.
Results
Description of Enrolled population
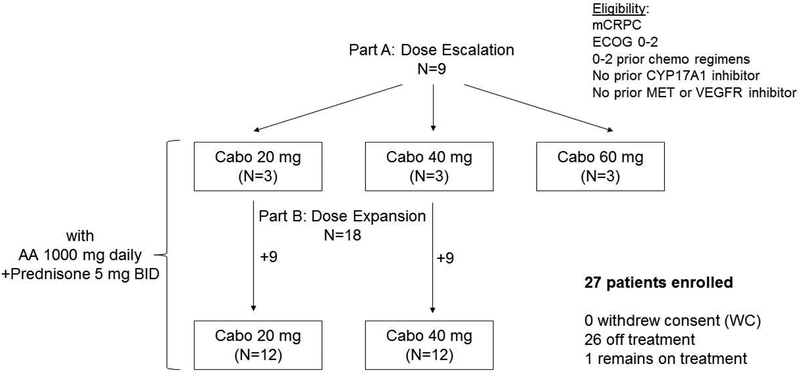
Between May 17, 2012, and May 27, 2014, the study enrolled total of the 27 patients. Nine patients were enrolled in the dose escalation phase (Part A) with 3 at each cabozantinib dose level of 20mg, 40mg and 60mg daily; an additional 9 patients were in each of the two expansion cohorts at 20mg and 40mg cabozantinib dose levels (Part B) (Figure 1). The median age was 64 (IQR, 61–70) and the median baseline PSA was 20 ng/mL (IQR 6–45). Eleven of 27 patients (40.7%) had previously received docetaxel chemotherapy, and 4/27 patients (14.8%) had visceral metastases (Table 1).
Figure 1.
Schema of patient cohorts in the dose escalation (Part A) and dose expansion (Part B) phases of this study. Cabo=cabozantinib, AA=abiraterone acetate.
Table 1.
Patient characteristics according to cabozantinib dose level
| Factor | Stat | cabozantinib, 20mg, N=12 | cabozantinib, 40mg, N=12 | cabozantinib, 60mg, N=3* | Total N=27 |
|---|---|---|---|---|---|
| Follow-up (months) | Median (IQR) | 21.8 (15.4,35.0) | 38.1 (17.1,41.8) | 55.8 (31.1,56.9) | 33.8 (16.9,43.2) |
| [3.6,60.4] | [7.4,58.5] | [6.3,58.0] | [3.6,60.4] | [3.6,60.4] | |
| Age at diagnosis (year) | Median (IQR) | 62 (59,66) | 60 (57,62) | 57 (55,59) | 60 (57,64) |
| Range | [50,74] | [49,68] | [53,61] | [49,74] | |
| Age at start of study (year) | Median (IQR) | 66 (61,72) | 62 (61,67) | 65 (60,68) | 64 (61,70) |
| Range | [52,76] | [53,75] | [56,71] | [52,76] | |
| Race (White) | N (%) | 12(100.0) | 11(91.7) | 3(100.0) | 26(96.3) |
| Baseline ECOG PS N (%) | 0 | 11(91.7) | 11(91.7) | 3(100.0) | 25(92.6) |
| 1 | 1(8.3) | 1(8.3) | 0(0.0) | 2(7.4) | |
| Gleason at Dx, N (%) | ≤6 | 0(0.0) | 1(8.3) | 1(33.3) | 2(7.4) |
| 7 | 2(16.7) | 2(16.7) | 0(0.0) | 4(14.8) | |
| 8–10 | 10(83.3) | 9(75.0) | 2(66.7) | 21(77.8) | |
| Baseline testosterone level (ng/dL) | Median (IQR) | 7 (0,10) | 11 (10,17) | 7 (7,7) | 9 (7,12) |
| Range | [0,16] | [7,24] | [7,7] | [0,24] | |
| Baseline PSA (ng/mL) | Median (IQR) | 31 (19,72) | 14 (2,47) | 8 (6,9) | 20 (6,45) |
| Range | [1,376] | [0,783] | [4,11] | [0,783] | |
| Prior docetaxel use | N (%) | 5(41.7) | 5(41.7) | 1(33.3) | 11(40.7) |
| Prior opiate use | N (%) | 5(41.7) | 6(50.0) | 1(33.3) | 12(44.4) |
| Visceral metastases | N (%) | 2(16.7) | 1(8.3) | 1(33.3) | 4(14.8) |
| LDH >1xULN, N (%) | Yes | 2(16.7) | 1(8.3) | 1(33.3) | 4(14.8) |
| No | 10(83.3) | 11(91.7) | 2(66.7) | 23(85.2) | |
| Normal Albumin (3.7–5.4 g/dL) | N (%) | 12(100.0) | 12(100.0) | 3(100.0) | 27(100.0) |
| HGB (<12.5 g/dL) N (%) | Low | 5(41.7) | 4(33.3) | 2(66.7) | 11(40.7) |
| Normal | 7(58.3) | 8(66.7) | 1(33.3) | 16(59.3) | |
| Alkaline Phosphatase (>118 U/L), N (%) | High | 4(33.3) | 4(33.3) | 0(0.0) | 8(29.6) |
| Normal | 8(66.7) | 8(66.7) | 3(100.0) | 19(70.4) |
Dose-limiting toxicities
There were no DLTs in first 4 weeks from the 3 dose levels in Part A (the dose escalation phase); however two of the 3 patients at the 60mg cabozantinib dose level required dose reduction beyond cycle 2 due to fatigue. For Part B, dose expansion was performed at the 20mg and 40mg dose levels given a more favorable long term tolerability profile compared to 60mg. An additional 18 patients were enrolled in Part B, 9 patients at each of the 20mg and 40mg dose levels, for a total of 12 patients treated at each of these doses including the patients treated in Part A. During the first cycle for the expansion cohorts, 0 of 9 patients experienced a DLT at the 20mg dose level, and 1 of 9 patients experienced a DLT (grade 3 asymptomatic lipase elevation requiring dose reduction) at the 40mg dose level. The 2-stage 90% exact binomial confidence intervals on the rate of DLTs are 0.0 (90%CI: 0.01–0.63) for the 20mg dose and 0.08 (90%CI: 0.02–0.86) for the 40mg dose.
Adverse events
Of the 27 patients included in the safety population, 13 patients (48%) experienced maximum grade 3 AEs, and 4 patients (15%) experienced grade 4 AEs: two from the cabozantinib 40mg cohort and two from the 60mg cohort (Table 2.1). All four grade 4 events (renal disorder and appendicitis at the 40mg dose level; aortic valve disease and hyperglycemia at the 60mg dose level) seen in this study were indicated by the treating investigator to be unrelated to abiraterone and cabozantinib. Twenty-six of 27 patients reported treatment related AEs (Table 2.2); 15 patients had maximum grade ≥3 treatment-related AEs of which 12 (80%) were felt related to cabozantinib: 3 patients at 20mg dose, 7 at 40mg dose and 2 at 60mg dose.
Table 2.1.
Number of Patients with ALL Adverse Events (AEs) according to cabozantinib dose level
| cabozantinib dose level | TOTAL | |||||||
|---|---|---|---|---|---|---|---|---|
| cabozantinib 20mg, abiraterone 1000mg |
cabozantinib 40mg, abiraterone 1000mg |
cabozantinib 60mg, abiraterone 1000mg |
||||||
| N | % | N | % | N | % | N | % | |
| Maximum Grade* | 2 | 16.7 | 1 | 8.3 | - | - | 3 | 11.1 |
| 1 | ||||||||
| 2 | 5 | 41.7 | 1 | 8.3 | 1 | 33.3 | 7 | 25.9 |
| 3 | 5 | 41.7 | 8 | 66.7 | - | - | 13 | 48.1 |
| 4 | - | - | 2 | 16.7 | 2 | 66.7 | 4 | 14.8 |
| Total | 12 | 100.0 | 12 | 100.0 | 3 | 100.0 | 27 | 100.0 |
maximum grade consolidates reports of all AEs of same type for a patient.
Table 2.2.
Number of Patients with Treatment-Related AEs according to cabozantinib dose level
| cabozantinib dose level | TOTAL | |||||||
|---|---|---|---|---|---|---|---|---|
| cabozantinib 20mg, abiraterone 1000mg |
cabozantinib 40mg, abiraterone 1000mg |
cabozantinib 60mg, abiraterone 1000mg |
||||||
| N | % | N | % | N | % | N | % | |
| Maximum Grade | 3 | 27.3 | 1 | 8.3 | - | - | 4 | 15.4 |
| 1 | ||||||||
| 2 | 3 | 27.3 | 3 | 25.0 | 1 | 33.3 | 7 | 26.9 |
| 3 | 5 | 45.5 | 8 | 66.7 | 2 | 66.7 | 15 | 57.7 |
| Total | 11 | 100.0 | 12 | 100.0 | 3 | 100.0 | 26 | 100.0 |
maximum grade consolidates reports of all AEs of same type for a patient.
The most common treatment-related grade ≥2 AEs (all cohorts) were hypertension (22.2%), hypophosphatemia (18.5%), elevated AST (11.1%), palmar-plantar erythrodysesthesia (11.1%) and musculoskeletal and connective tissue disorders (11.1%: 1 herniated disc, 2 muscle weakness). Treatment-related Grade 3 AEs experienced by more than one patient (all cohorts) included infections (11.1%), hypophosphatemia (11.1%), thromboembolic events (n=2), anemia (n=2), elevated lipase (n=2), and elevated ALT (n=2) (Table 2.3). A total of 14 of 27 (52%) of patients on trial had cabozantinib dose reductions or discontinuations due to toxicity: 3 of 12 at the 20mg starting dose, 8 of 12 at the 40mg starting dose, and 3 of 3 at the 60mg starting dose.
Table 2.3.
Frequency and proportion of most commonly reported AEs that were indicated as possibly, probably, or definitely related to protocol therapy
| Maximum Grade 2+ (with N>1 patients) | N (%) | Maximum Grade 3 (with N>1 patients) | N (%) |
|---|---|---|---|
| Hypertension | 6(22) | Infection | 3(11) |
| Hypophosphatemia | 5(19) | Hypophosphatemia | 3(11) |
| AST | 3(11) | Anemia | 2(7) |
| Palmar-plantar erythrodysesthesia syndrome | 3(11) | ALT | 2(7) |
| Musculoskeletal and connective tissue | 3(11) | Lipase increase | 2(7) |
| Anemia | 2(7) | Thromboembolic event | 2(7) |
| ALT | 2(7) | ||
| Thromboembolic event | 2(7) |
Pharmacokinetics
The geometric mean Cminss for abiraterone 1,000 mg QD was similar in patients receiving cabozantinib at doses of 20 mg QD (14.1 ± 4.5 ng/mL, n=11) and 40 mg QD (15.2 ± 7.6 ng/mL, n=8). Data was available for only 2 patients treated with 60 mg cabozantinib precluding meaningful comparisons with data from the other two dose levels. The median Cminss for abiraterone for all 21 patients was 16.0 ng/mL (range, 5.9–29.8 ng/mL). The geometric mean Cminss for cabozantinib given once daily was 213 ± 109 ng/mL for the 20 mg QD dose (n=11) and 434 ± 146 ng/mL for patients treated with 40 mg QD (n=8). The geometric mean dose-normalized Cminss of cabozantinib for all 21 patients was 11.1 ± 4.9 ng/mL/mg.
Description of efficacy results
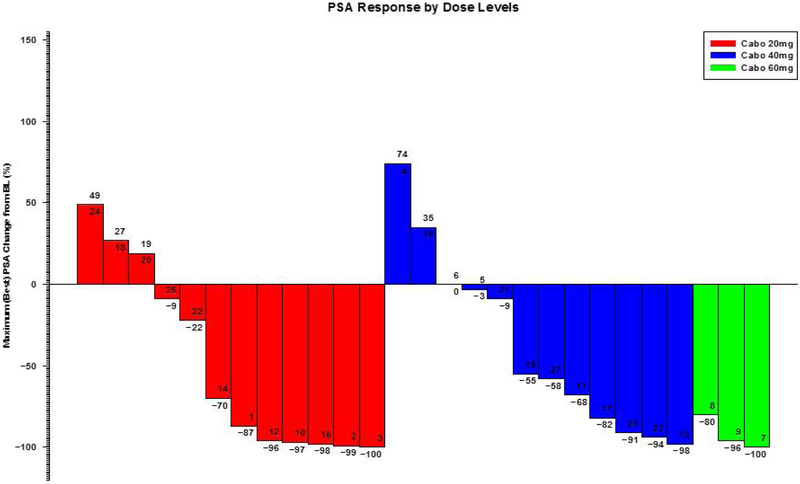
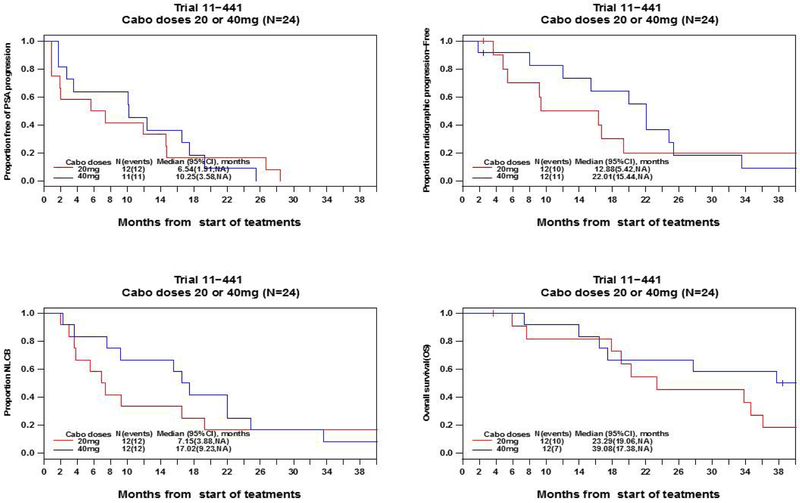
As of data lock October 24, 2017, twenty-six (26) patients had been discontinued from study treatment, while 1 patient remained on treatment. The median follow-up was 33.8 months (IQR: 16.9–43.2 months). Maximum PSA declines of >50% were seen in 58.3%, 58.3% and 100% of the patients in the 20mg, 40mg and 60mg cohorts, respectively (Figure 2). For the patients treated at the 20mg and 40mg dose levels, Kaplan-Meier curves for time to PSA progression, time to radiographic progression, time to progression (by earlier of radiographic progression or “no longer clinically benefiting”28), and overall survival are shown in Figure 3. The estimated median time to radiographic progression was 12.88 months (95% CI 5.42 – not estimated [NE]) in the 20mg cohort and 22.01 months (95% CI 15.44 - NE) in the 40mg cohort, and median overall survival was 23.29 months (95% CI 19.06 - NE) in the 20mg cohort and 39.08 months (95% CI 17.38 - NE) in the 40mg cohort (95% CI 0.21 – 1.53). Based on tolerability and preliminary efficacy, abiraterone acetate 1000mg daily with cabozantinib 40mg daily is the recommended phase 2 dose (RP2D).
Figure 2.
Waterfall plot of maximal PSA change (%) from baseline by cabozantinib dose level.
Figure 3.
Kaplan-Meier curves for A. time to PSA progression B. time to radiographic progression C. time to progression by the earlier of radiographic progression or no longer clinically benefiting (NLCB) and D. overall survival in patients treated at 20mg and 40mg dose levels.
Discussion
The development of rational therapeutic combinations to enhance the anti-tumor activity and prolong clinical benefit from abiraterone acetate treatment is an important unmet clinical need in prostate cancer. Here we demonstrate that abiraterone can be safely combined with cabozantinib in patients with mCRPC. Though there were no dose-limiting toxicities for cabozantinib 20mg, 40mg or 60mg at 4 weeks after starting treatment, the 40mg and 20mg doses were favored for dose expansion given better long-term tolerability compared to cabozantinib 60mg. While 10 of the 12 patients treated at the 40mg dose experienced grade 3 or higher toxicities, these were mostly asymptomatic (such as hypertension and alteration in laboratory parameters) and were easily managed pharmacologically or with dose delays and/or reductions. None of the grade 4 toxicities seen in this study were felt to be related to abiraterone or cabozantinib, and there was no treatment-related mortality. There was a suggestion of greater clinical benefit with the 40mg dose as compared to the 20mg dose – while the initial PSA response rate to both doses was similar, there was a trend towards longer time to progression and overall survival with the 40mg dose. These findings are in consonance with a prior study suggesting greater pharmacodynamic activity of 40mg compared to 20mg with respect to bone scan response.29 Moreover, patients treated with 40mg of cabozantinib with abiraterone had longer median radiographic progression free-survival (22.01 months) and overall survival (34.7 months) versus the outcome seen in patients treated with abiraterone alone in the COU-AA-302 study (16.5 months and 39.08 months respectively)30,31 despite the fact that our study allowed patients with visceral metastases and prior chemotherapy exposure. These poorer prognosis patients were excluded from the COU-302 trial. Our findings suggest that combining cabozantinib at 40mg with abiraterone was tolerable and could lead to prolonged benefit compared to abiraterone alone.
The development of abiraterone acetate as a therapeutic agent in prostate cancer has been transformative to management of these patients. With results of the LATITUDE10 and STAMPEDE11 trials demonstrating a survival benefit to the addition of abiraterone to initial androgen deprivation therapy for metastatic hormone-sensitive prostate cancer, the contexts in which meaningful clinical benefit from abiraterone have been demonstrated continue to expand. A major ongoing research effort is focusing on incorporating abiraterone acetate in treatment paradigms for localized disease, including concurrent treatment with external beam radiation therapy32, or in the neoadjuvant33,34 and neoadjuvant/adjuvant (NCT02903368) settings. If abiraterone decreases metastatic relapse in these settings, this will lead to further decreases in the death rate attributable to prostate cancer35, as well as decrease suffering related to symptoms of recurrent disease and its treatment.
In this context, elucidating and therapeutic targeting of resistance mechanisms to abiraterone are critical to further enhance clinical benefits derived from this agent. Specifically, resistance to abiraterone could involve compensatory signaling through alternate survival pathways, and co-targeting of these pathways would be expected to lead to superior clinical outcomes, as has been demonstrated with the addition of the MEK inhibitor trametinib to the RAF inhibitor dabrafenib in the first line treatment of BRAF-mutated melanoma36,37.
Cabozantinib is an intriguing partner for combination treatment with abiraterone given its mechanism of action. As a multi-kinase inhibitor, it can simultaneously target critical signaling pathways as well as compensatory pathways. For example, inhibition of compensatory upregulation of MET signaling with blockade of the VEGF pathway has been implicated as its major mechanism of action in renal cell carcinoma38,39. Two of the major targets of cabozantinib, MET and VEGFR2, have both been mechanistically implicated in the development of castration resistance in patients40. Interestingly, in a PTEN/p53-deficient genetically engineered mouse model, the anti-tumor activity of cabozantinib was demonstrated not to be related to inhibition of MET but rather to activation of a neutrophil-mediated anticancer innate immune response41. In a preclinical model of prostate cancer, it has been demonstrated that cabozantinib can inhibit compensatory upregulation of the IGF1R/MEK/ERK pathway in prostate cancer cells induced by abiraterone treatment18. Clearly, further investigation is needed to clarify the mechanism by which cabozantinib may enhance the activity of abiraterone in patients.
Cabozantinib demonstrated interesting single-agent clinical activity in a Phase 2 nonrandomized expansion study in castration-resistant prostate cancer (CRPC), demonstrating bone scan response rates of 73% and 45% in 100mg (N=93) and 40mg (N=51) cohorts, respectively.16 In addition, there were improvements seen in measurable soft tissue disease, circulating tumor cells and bone biomarkers, along with improvements in pain and analgesic use.42 In these studies, the 100mg dose of cabozantinib was associated with significant toxicity so a 60mg dose was chosen for the subsequent Phase 3 COMET-1 study, where patients were randomized to cabozantinib vs. prednisone with primary endpoint of overall survival. The COMET-1 study demonstrated improvements in bone scan response, radiographic progression-free survival, symptomatic skeletal events, CTC conversions, and bone biomarkers but not PSA outcomes or overall survival. Because the COMET-1 study did not demonstrate an OS benefit, the Phase 3 COMET-2 study comparing pain palliation with cabozantinib vs. a control arm of mitoxantrone/prednisone was discontinued early. Final results of COMET-2 demonstrated that in the 119 patients randomized prior to discontinuation, pain response rates were 15% for cabozantinib and 17% for mitoxantrone/prednisone; bone scan response rates were 31% for cabozantinib and 5.2% for mitoxantrone/prednisone.43 Why the COMET-1 and COMET-2 studies demonstrated improvements in intermediate parameters but not their primary endpoints remains unclear. One possibility is that these intermediate parameters do not reflect a change in disease biology that leads to prolongation of survival. In addition, we hypothesize that part of the reason that the Phase 3 studies did not meet their primary endpoints is that these patients were heavily pre-treated with both taxane and androgen-receptor targeted therapies (all patients were required to have failed docetaxel as well as abiraterone and/or enzalutamide), whereas in the Phase 2 study cited above16 only 44% of patients had received prior abiraterone and 4% received prior enzalutamide. It has been suggested that upregulation of c-Met is an early compensatory event with androgen ablation44 that can be therapeutically targeted with inhibitors of c-Met.45 These studies would suggest that agents that inhibit the MET pathway, such as cabozantinib, may have increased activity earlier in the disease course, particularly in combination with androgen-receptor targeted therapy.
CYP3A4/5 is the major cytochrome P450 (CYP) isozyme involved in the hepatic metabolism of both abiraterone and cabozantinib in humans, with minimal involvement of other CYP isozymes.24,46 In vitro studies using human hepatic microsomes showed that abiraterone is a moderate inhibitor of CYP3A4/5 and that cabozantinib inhibits the isozyme weakly. Cabozantinib is not a potent inducer of CYP3A4/5 and the induction potential of abiraterone on CYP isozymes has not been reported. The pharmacokinetics of cabozantinib are readily altered by agents that significantly inhibit or induce CYP3A4 activity.21 Systemic exposure to abiraterone was decreased by 55% when given to subjects that had been pretreated with the strong CYP3A4 inducer rifampin but not significantly affected when coadministered with the strong CYP3A4 inhibitor ketoconazole.47 The plasma pharmacokinetics of erlotinib, an approved anticancer drug for which CYP3A4 metabolism represents a prominent route of its elimination, was not affected by the coadministration of cabozantinib in a phase I study in patients with solid tumors.24 The current understanding of the pharmacokinetics and metabolism of abiraterone and cabozantinib therefore suggests that there is a relatively low potential for a clinically relevant alteration in the plasma pharmacokinetics of either drug when given concurrently.
Two different dosage forms for oral administration have been used during the course of the clinical development of cabozantinib, the original capsule and a newer tablet formulation. While publically accessible information on the Cminss of cabozantinib is available from several clinical trials in which patients with solid tumors received 140 mg QD doses of the capsule formulation,48 similar data is not available for the tablet formulation used in this trial. The two dosage forms are not strictly bioequivalent, primarily due to differences in the rate of absorption following administration of a single 140 mg dose that gives rise to statistically different maximum concentrations of the drug in plasma, although they otherwise provide nearly indistinguishable plasma concentration-time curves when administered over a broad range of clinically relevant doses.49 Linear pharmacokinetics has been demonstrated for the tablet formulation at single doses ranging from 20–140 mg. Thus, although a formal comparison of the steady state pharmacokinetics for the two dosage forms have not been undertaken, there appears to be sufficient justification to use dose-normalized Cminss values to compare data from the present study with data from prior clinical trials of single agent cabozantinib that employed the capsule formulation.
Consistent with the known linear pharmacokinetic behavior of cabozantinib for the tablet dosage form,49 the geometric mean Cminss of cabozantinib in patients treated with doses of 40 mg QD (434 ± 146 ng/mL, n=11) was 2.04 times greater than in patients treated with 20 mg QD (213 ± 109 ng/mL, n=8), when given together with abiraterone 1,000 mg QD. The geometric mean (±SD) dose-normalized Cminss of cabozantinib (i.e. Cminss divided by dose in mg) for all 21 patients with evaluable data in the current study, 11.1 ± 4.9 ng/mL/mg, was within the 7.7 −34.1 ng/mL/mg range of dose-normalized mean Cminss values of the drug in CRPC patients treated with 100 mg QD of the capsule formulation as a single agent (data on file at Exelixis). These findings suggest that the steady state pharmacokinetics of cabozantinib were not affected by the co-administration of abiraterone.
The overall median Cminss of abiraterone in the present study for the entire group of 21 patients with evaluable pharmacokinetic data was 16.0 ng/mL (range, 5.9–29.8 ng/mL), compared to 11.1 ng/mL (range not reported) in 119 patients evaluated in the Phase 3 COU-AA-301 trial46 and 10.5 ng/mL (range, 2.6–26.9 ng/mL) over the first 3 months of treatment in a group of 55 patients in another recently reported study.50 The range of abiraterone concentrations seen in combination with cabozantinib in our study overlaps with ranges of Cminss in previous reports. The median abiraterone concentration seen in our study is numerically different from prior studies, though it is entirely possible that other unknown factors, such as differences in patient characteristics or their diets, the time that samples were collected relative to the prior dose, consistency in the daily dosing time, the analytical methods used to measure the drug concentration, or interpatient variability in the pharmacokinetics of the drug, contributed to the differences seen here. A more formal drug-drug interaction study would be needed to definitively determine if cabozantinib affects abiraterone levels. If cabozantinib were shown to mitigate the likelihood of low systemic abiraterone exposure in some patients, this could also increase the chance of clinical benefit as low abiraterone levels have previously been correlated with inferior outcomes.50,51 However, there is no evidence that this is the mechanism mediating potentially greater clinical benefit seen at the 40mg dose level in this study compared to the 20mg dose level as the abiraterone concentrations were nearly equivalent in these groups: 14.1 ± 4.5 ng/mL (n=11) at the 20mg dose and 15.2 ± 7.6 ng/mL (n=8) at the 40mg dose.
In conclusion, we have demonstrated safety, tolerability, and preliminary evidence of clinical activity with the combination of abiraterone and cabozantinib. The results of this trial provide important information to guide the design of a definitive comparative trial, which will more fully assess the ability of cabozantinib to increase duration of cancer control and overall survival in combination with abiraterone in patients with prostate cancer.
Acknowledgements:
This study is an Investigator Sponsored Trial supported by Exelixis, Inc. This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Disclosure Statement:
Choudhury AD. Honoraria: Bayer, Astellas, Janssen. Research Funding: Bayer (pending)
Harshman LC. Advisory: Bayer, Genentech, Dendreon, Pfizer, Medivation/Astellas, Kew Group, Theragene, Corvus, Merck, Exelixis; Research Funding (Inst): Bayer, Sotio, Bristol-Myers Squib, Merck, Takeda, Dendreon/Valient, Jannsen, Medivation/Astellas, Genentech, Pfizer; CME: PER, Applied Clinical Education
Taplin ME. Advisory: Janssen, Research Funding: Janssen
Bernard B. Honoraria: Astellas
Kantoff PW. Advisory: BIND Biosciences, Inc., BN Immunotherapeutics, Context Therapeutics LLC, DRGT, GE Healthcare, Janssen, Metamark, New England Research Institutes Inc., OncoCellMDX, Progenity, Sanofi, Seer Biosciences, Tarveda Therapeutics (previously Blend) , Thermo Fisher; Investment Interest: Context Therapeutics LLC, DRGT, Seer Biosciences, Tarveda Therapeutics (previously Blend); Data Safety Monitoring Board: Genentech/Roche, Merck
Sweeney C. Advisory: Sanof i, Janssen Biotech, Astellas Pharma, Bayer, Genentech, AstraZeneca, Pfizer; Research Funding (Inst): Janssen Biotech, Astellas Pharma, Sanofi, Bayer, Sotio; Patents, Royalties, Other Intellectual Property: Leuchemix, Parthenolide, Dimethylaminoparthenolide; Exelixis: Abiraterone plus cabozantinib combination; Investment Interest: Leuchemix
References
- 1.Key Statistics for Prostate Cancer. https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html, 2017
- 2.Pienta KJ, Bradley D: Mechanisms underlying the development of androgen-independent prostate cancer. Clin Cancer Res 12:1665–71, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Nakazawa M, Paller C, Kyprianou N: Mechanisms of Therapeutic Resistance in Prostate Cancer. Curr Oncol Rep 19:13, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards J, Bartlett JM: The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer. Part 1: Modifications to the androgen receptor. BJU Int 95:1320–6, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Taplin ME, Balk SP: Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem 91:483–90, 2004 [DOI] [PubMed] [Google Scholar]
- 6.O’Donnell A, Judson I, Dowsett M, et al. : Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer 90:2317–25, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai C, Chen S, Ng P, et al. : Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res 71:6503–13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bono JS, Logothetis CJ, Molina A, et al. : Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364:1995–2005, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan CJ, Smith MR, de Bono JS, et al. : Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 368:138–48, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fizazi K, Tran N, Fein L, et al. : Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. New England Journal of Medicine 0:null [DOI] [PubMed] [Google Scholar]
- 11.James ND, de Bono JS, Spears MR, et al. : Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. New England Journal of Medicine 0:null [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galletti G, Leach BI, Lam L, et al. : Mechanisms of resistance to systemic therapy in metastatic castration-resistant prostate cancer. Cancer Treat Rev 57:16–27, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Nelson PS: Molecular states underlying androgen receptor activation: a framework for therapeutics targeting androgen signaling in prostate cancer. J Clin Oncol 30:644–6, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Sennino B, Naylor RM, Tabruyn SP, et al. : Abstract A13: Reduction of tumor invasiveness and metastasis and prolongation of survival of RIP-Tag2 mice after inhibition of VEGFR plus c-Met by XL184, AACR, 2009 [Google Scholar]
- 15.You WK, Sennino B, Williamson CW, et al. : VEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancer. Cancer Res 71:4758–68, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bluemn EG, Coleman IM, Lucas JM, et al. : Androgen Receptor Pathway-Independent Prostate Cancer Is Sustained through FGF Signaling. Cancer Cell 32:474–489 e6, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith MR, Bono JSD, Sternberg CN, et al. : Final analysis of COMET-1: Cabozantinib (Cabo) versus prednisone (Pred) in metastatic castration-resistant prostate cancer (mCRPC) patients (pts) previously treated with docetaxel (D) and abiraterone (A) and/or enzalutamide (E). Journal of Clinical Oncology 33:139–139, 2015 [Google Scholar]
- 18.Wang X, Huang Y, Christie A, et al. : Cabozantinib Inhibits Abiraterone’s Upregulation of IGFIR Phosphorylation and Enhances Its Anti-Prostate Cancer Activity. Clin Cancer Res 21:5578–87, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Martins V, Asad Y, Wilsher N, et al. : A validated liquid chromatographic-tandem mass spectroscopy method for the quantification of abiraterone acetate and abiraterone in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 843:262–7, 2006 [DOI] [PubMed] [Google Scholar]
- 20.US Food and Drug Administration CfDEaR: Guidance for Industry, Bioanalytical Method Validation, 2001 [Google Scholar]
- 21.Nguyen L, Holland J, Miles D, et al. : Pharmacokinetic (PK) drug interaction studies of cabozantinib: Effect of CYP3A inducer rifampin and inhibitor ketoconazole on cabozantinib plasma PK and effect of cabozantinib on CYP2C8 probe substrate rosiglitazone plasma PK. J Clin Pharmacol 55:1012–23, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Attard G, Reid AH, Yap TA, et al. : Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol 26:4563–71, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Tolcher AW, Chi KN, Shore ND, et al. : Effect of abiraterone acetate plus prednisone on the QT interval in patients with metastatic castration-resistant prostate cancer. Cancer Chemother Pharmacol 70:305–13, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacy SA, Miles DR, Nguyen LT: Clinical Pharmacokinetics and Pharmacodynamics of Cabozantinib. Clin Pharmacokinet 56:477–491, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Miller RG: The jackknife-a review. Biometrika 61:1–15, 1974 [Google Scholar]
- 26.Scher HI, Halabi S, Tannock I, et al. : Design and End Points of Clinical Trials for Patients With Progressive Prostate Cancer and Castrate Levels of Testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. Journal of Clinical Oncology 26:1148–1159, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atkinson EN, Brown BW: Confidence limits for probability of response in multistage phase II clinical trials. Biometrics:741–744, 1985 [PubMed] [Google Scholar]
- 28.Scher HI, Morris MJ, Stadler WM, et al. : Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. Journal of Clinical Oncology 34:1402–1418, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee RJ, Saylor PJ, Michaelson MD, et al. : A dose-ranging study of cabozantinib in men with castration-resistant prostate cancer and bone metastases. Clin Cancer Res 19:3088–94, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rathkopf DE, Smith MR, de Bono JS, et al. : Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol 66:815–25, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan CJ, Smith MR, Fizazi K, et al. : Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 16:152–60, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Cho E, Mostaghel EA, Russell KJ, et al. : External beam radiation therapy and abiraterone in men with localized prostate cancer: safety and effect on tissue androgens. Int J Radiat Oncol Biol Phys 92:236–43, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Efstathiou E, Davis JW, Troncoso P, et al. : Cytoreduction and androgen signaling modulation by abiraterone acetate (AA) plus leuprolide acetate (LHRHa) versus LHRHa in localized high-risk prostate cancer (PCa): Preliminary results of a randomized preoperative study. Journal of Clinical Oncology 30:4556–4556, 2012 [Google Scholar]
- 34.Taplin ME, Montgomery B, Logothetis CJ, et al. : Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. J Clin Oncol 32:3705–15, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie W, Regan MM, Buyse M, et al. : Metastasis-Free Survival Is a Strong Surrogate of Overall Survival in Localized Prostate Cancer. J Clin Oncol 35:3097–3104, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Long GV, Stroyakovskiy D, Gogas H, et al. : Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 371:1877–88, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Flaherty KT, Infante JR, Daud A, et al. : Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 367:1694–703, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu SS, Quinn DI, Dorff TB: Clinical use of cabozantinib in the treatment of advanced kidney cancer: efficacy, safety, and patient selection. Onco Targets Ther 9:5825–5837, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choueiri TK, Escudier B, Powles T, et al. : Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 17:917–27, 2016 [DOI] [PubMed] [Google Scholar]
- 40.Aftab DT, McDonald DM: MET and VEGF: synergistic targets in castration-resistant prostate cancer. Clin Transl Oncol 13:703–9, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patnaik A, Swanson KD, Csizmadia E, et al. : Cabozantinib Eradicates Advanced Murine Prostate Cancer by Activating Antitumor Innate Immunity. Cancer Discov 7:750–765, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basch E, Autio KA, Smith MR, et al. : Effects of cabozantinib on pain and narcotic use in patients with castration-resistant prostate cancer: results from a phase 2 nonrandomized expansion cohort. Eur Urol 67:310–8, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Basch EM, Scholz MC, Bono JSD, et al. : Final analysis of COMET-2: Cabozantinib (Cabo) versus mitoxantrone/prednisone (MP) in metastatic castration-resistant prostate cancer (mCRPC) patients (pts) with moderate to severe pain who were previously treated with docetaxel (D) and abiraterone (A) and/or enzalutamide (E). Journal of Clinical Oncology 33:141–141, 2015. 25185099 [Google Scholar]
- 44.Verras M, Lee J, Xue H, et al. : The androgen receptor negatively regulates the expression of c-Met: implications for a novel mechanism of prostate cancer progression. Cancer Res 67:967–75, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Tu WH, Zhu C, Clark C, et al. : Efficacy of c-Met inhibitor for advanced prostate cancer. BMC Cancer 10:556, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.US Food and Drug Administration CfDEaR, NDA 202–379: Clinical Pharmacology and Biopharmaceutics Review - Abiraterone Acetate, 2011 [Google Scholar]
- 47.Bernard A, Vaccaro N, Acharya M, et al. : Impact on abiraterone pharmacokinetics and safety: Open-label drug-drug interaction studies with ketoconazole and rifampicin. Clin Pharmacol Drug Dev 4:63–73, 2015 [DOI] [PubMed] [Google Scholar]
- 48.US Food and Drug Administration CfDEaR: Clinical Pharmacology and Biopharmaceutics Review(s), NDA 203756, Cabozantinib, 2012 [Google Scholar]
- 49.Nguyen L, Benrimoh N, Xie Y, et al. : Pharmacokinetics of cabozantinib tablet and capsule formulations in healthy adults. Anticancer Drugs 27:669–78, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Carton E, Noe G, Huillard O, et al. : Relation between plasma trough concentration of abiraterone and prostate-specific antigen response in metastatic castration-resistant prostate cancer patients. Eur J Cancer 72:54–61, 2017 [DOI] [PubMed] [Google Scholar]
- 51.Friedlander TW, Graff JN, Zejnullahu K, et al. : High-Dose Abiraterone Acetate in Men With Castration Resistant Prostate Cancer. Clin Genitourin Cancer 15:733–741 e1, 2017 [DOI] [PubMed] [Google Scholar]