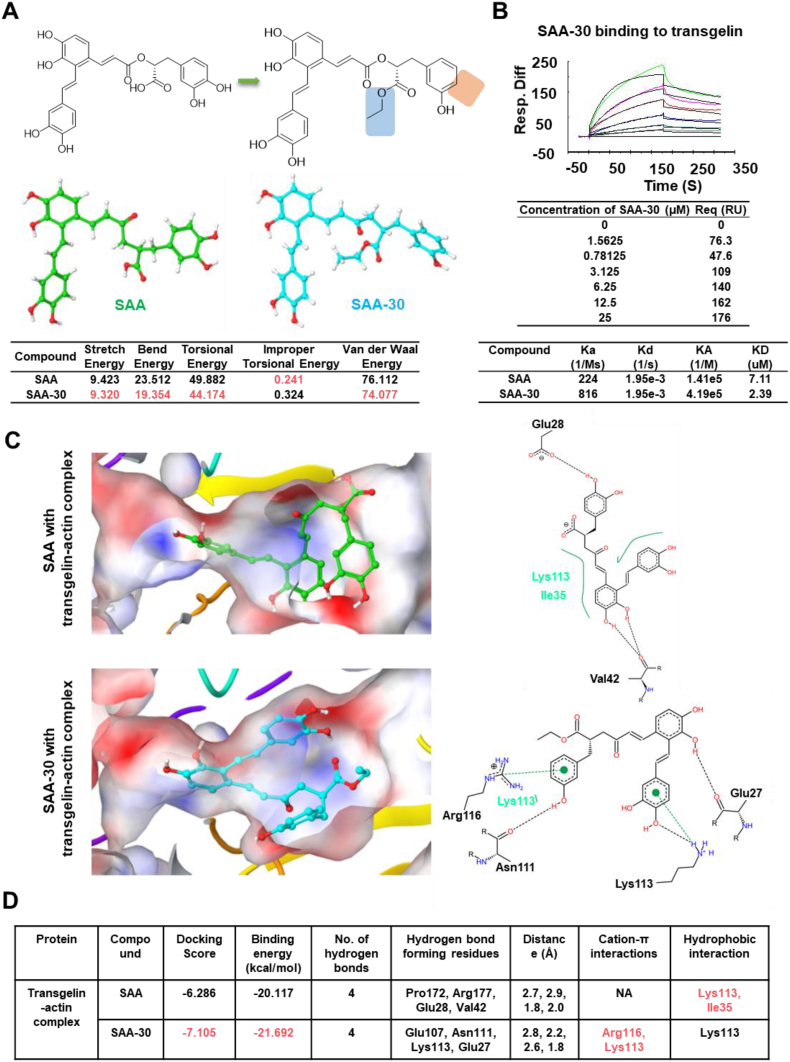
Fig. 4.
Comparison of SAA and SAA-30 structural and binding modes. (A) The structural energy levels of SAA-30 was lower than that of SAA. (B) Biacore results for SAA-30 bound to transgelin. SAA-30 was three times more active than SAA. (C) The binding mode of SAA and SAA-30 in the active site of the transgelin and actin complex. SAA-30 generated a higher docking score of −7.105 relative to that of the transgelin-actin complex. The green dotted line denotes the cation-π interaction. The black dotted line denotes hydrogen bond interactions, and the green solid line denotes hydrophobic interactions. (D) Table showing docking scores, binding energy levels, hydrogen bond interactions, cation-π interactions and hydrophobic interactions between SAA and SAA-30 and the transgelin-actin complex. SAA-30 shows lower binding energy levels in the transgelin-actin complex than in the SAA.