Abstract
Background
Osteonecrosis of the knee (ONK) is a form of aseptic necrosis resulting from ischemia to subchondral bone tissue. Typically, treatment is invasive. Hyperbaric oxygen therapy (HBOT) may provide a noninvasive alternative by improving oxygenation and reperfusion of ischemic areas. This study evaluates the efficacy of HBOT in a series of ONK patients.
Methods
This retrospective study evaluates 37 ONK patients (29 male, 8 female; mean age ± 1 standard deviation: 54 ± 14); 83.7% of patients presented with Aglietti stage I-II; 16.3% presented with Aglietti stage III. Patients were treated with HBOT once a day, 5 days a week, at 2.5 atmosphere absolute with 100% inspired oxygen by mask for an average of 67.9 ± 15 sessions. Magnetic resonance imaging was performed before HBOT, within 1 year after completion of HBOT, and in 14 patients, 7 years after treatment. Oxford Knee Scores (OKSs) were recorded before HBOT and at the end of each HBOT treatment cycle.
Results
After the 30 sessions of HBOT, 86% of patients experienced improvement in their OKS, 11% worsened, and 3% did not change. All patients improved in OKS after 50 sessions. Magnetic resonance imaging evaluation 1 year after HBOT completion showed that edema at the femoral condyle had resolved in all but 1 patient.
Conclusions
HBOT is beneficial for treating ONK. Patients experienced improvements in pain and mobility as demonstrated by improvement in OKS. Radiographic improvements were also seen upon post-treatment follow-up. Aglietti staging for the entire sample saw an aggregate decrease (P < .01) from 1.7 ± 0.7 to 0.3 ± 0.6.
Keywords: Aseptic necrosis, Femoral condyles, Hyperbaric oxygen therapy (HBOT), Osteonecrosis, MRI
Introduction
The knee is the second most frequent location for aseptic osteonecrosis after the femoral head. Despite the high prevalence, literature regarding this disease process and its treatment remains limited in size and scope [1], [2], [3]. Osteonecrosis is a debilitating ischemic disease process that results in localized pain and tissue death of subchondral bone [2], [3]. While initially synovial fluid is able to keep the overlaying cartilage intact, the underlying necrotic tissue will eventually result in an inflammatory response that damages articular surfaces. If untreated the decrease in blood supply to the load-bearing bone structure of joints can result in joint collapse. Without early recognition and proper management of the disease, invasive procedures such as total knee replacement may be necessary [1], [2], [4].
Current characterization and staging for osteonecrosis of the knee (ONK) is often performed using the 5 stages of the Aglietti Radiographic Scale. The Aglietti Scale evaluates radiographic characteristics of the knee and, if applicable, measures the area of the necrotic lesion by multiplying the greatest width in the anteroposterior view by the greatest length in the lateral view [5]. Although this is a valuable tool for prognosis and plan of care, it is a poor tool for early diagnosis as radiographic evidence of osteonecrosis is often absent in early stages of the disease. Generally, early Aglietti staging (I-II) is associated with reversibility of the disease process. This can be accomplished through reperfusion and oxygenation of the ischemic tissue. Invasive interventions described include core decompression, bone grafting, and tibial osteotomy [6]. Post-collapse cases require more radical operations like total knee arthroplasty [1], [6]. To date, several noninvasive therapies have been evaluated to increase tissue perfusion and oxygenation including the use of extracorporeal shockwave therapy, anticoagulants, vasoactive substances, and hyperbaric oxygen therapy (HBOT) [7], [8].
Oxygen breathing (100%) in a hyperbaric chamber, or HBOT, has been shown to improve tissue oxygenation in ischemic and pre-necrotic areas [9], [10], [11]. HBOT increases the partial pressure of oxygen, which as stated in Henry's law is directly proportional to the amount of oxygen dissolved in blood plasma, (PO2:[O2] = α PO2), where α is the solubility constant of blood plasma and PO2 is the partial pressure of oxygen. Increased plasma O2 allows for greater tissue oxygenation. In addition, HBOT has been shown to increase the level of reactive oxygen species in tissue [11]. Reactive oxygen species can trigger a set of cellular responses that improve neovascularization and modulate impaired proinflammatory cytokine production [9], [10], [11]. While repetitive exposure to HBOT may induce otic barotrauma in approximately 10% of patients, this is a tolerable side effect which can be managed with decongestants. Although aseptic necrosis of bone the primary disease process of ONK, has yet to be approved as an indication by the Undersea and Hyperbaric Medical Society [9], [12], we described successful utilization of HBOT in early stages of avascular necrosis of the femoral head (AVNF) [9], [12], [13]. Thus, we reasoned that HBOT can also be used effectively in conservative management of ONK. This study aims to evaluate the efficacy of HBOT as a treatment modality for ONK.
Material and methods
This study comprises a retrospective chart review of patients with ONK; the institutional review board approval was obtained through the Local Ethics Committee of the University of Padova, Italy. The cohort of patients described in this study was treated with HBOT per criteria accepted as appropriate by the local health consortium, USL-5 in Fidenza, Italy. In this region of northern Italy, HBOT has been successfully used to alleviate symptoms of ONK. Patient enrollment dates range from November 1999 to February 2012. Patients received treatment after referral by their orthopaedic surgeon. The prevalent presenting symptom in this cohort was unilateral knee pain. Patients included in the study had no history of trauma, knee arthroplasty, or steroid use. Patients who accepted for treatment received a physical evaluation, pain assessment, plain anteroposterior and lateral knee radiograph, and magnetic resonance imaging (MRI). Owing to our resource limitations, the time between a patient's initial visit and completion of the first MRI reached 102 days on average. Patients who received MRI were staged using the Aglietti Scale as described: stage I, radiographs appear normal; stage II, flattening of the condyle can be visualized (lesion size 0-2.0 cm2); stage III, a radiolucent lesion of the subchondral bone can be seen (lesion size 2.1-4.5 cm2); stage IV, perifocal sclerosis is evident, and the subchondral bone has collapsed and is visibly calcified (lesion size 4.6-6.0 cm2); and stage V, secondary degenerative changes are present on both femur and tibia such as osteophyte formation, subchondral sclerosis, and visible bone erosion (lesion size ≥6.1 cm2). MRIs, and therefore Aglietti staging, were obtained before HBOT, after completion of HBOT (within 1 year after initiating HBOT), and 7 years after HBOT.
Pain was assessed using the Oxford Knee Evaluation, a survey consisting of 12 questions regarding patient subjective pain and active range of motion. The survey was used to determine a pretreatment Oxford Knee Score (OKS) for the patient. Traditionally, when grading an OKS, a score of 0 to 19 indicates severe knee arthritis and suggests the need for surgical intervention. A score of 20-29 indicates moderate-to-severe knee arthritis and suggests the need for an orthopaedic consult. A score of 30-39 indicates mild-to-moderate knee arthritis and suggests conservative management through moderate exercise, anti-inflammatory drugs, and weight loss. A score of 40-60 indicates normal joint function. The OKS was subsequently used to track treatment progression. Oxford knee evaluations were taken after each cycle of HBOT treatments.
The HBOT protocol used had been previously developed to treat AVNF, and has shown reproducible results [8], [9]. Patients were placed inside a multiplace hyperbaric chamber where they were compressed at 2.5 atmosphere absolute, once a day, 5 days a week. During compression at 2.5 atmosphere absolute, patients were exposed to 100% inspired oxygen by mask for 60 minutes. The mask was tightly sealed to ensure 100% oxygen was being delivered from the mask to the patient. The total length of the procedure including compression and decompression was 82 minutes. To minimize risk of flammability, the gas composition in the hyperbaric chamber was sampled every 5 minutes to detect any mask leakages and maintain a chamber oxygen gas composition of <23%. Ultimately, patients were to receive up to 4 cycles of treatment totaling 90 sessions. The first cycle consisted of 30 sessions, Monday to Friday for 6 weeks. After a 2-month break, patients received a second cycle consisting of 20 more sessions Monday to Friday for 4 weeks. The third and fourth cycles were identical, following a month break, and patients received 20 additional sessions Monday to Friday for 4 weeks. No additional treatments were used; patients were advised to avoid overly vigorous exercise.
Results
Demographics
The study population consisted of 29 males and 8 females with a mean age of 54 ± 13 years (Table 1). On average, patients received 68 treatments over 3 cycles (cycle 1: 30, cycle 2: 20, cycle 3: 20); only 28 subjects completed the last cycle of treatments. Localization of the necrotic lesion on the femoral condyles was characterized for the sample using radiographic imaging (Table 1); most patients' lesions were localized on the medial condyle (56.4%). There was no significant difference in treatment susceptibility based on lesion localization.
Table 1.
Patient demographics and HBO treatment overview.
| Gender | 29 M; 8 F |
| Average age (±standard deviation) | 54 ± 13 |
| Number of treatments | 67.9 ± 15 |
| Average number of cycles | 3.2 |
| Lesion localization by MRI | |
|---|---|
| Location | Percentage |
| Lateral | 43.6% |
| Medial | 56.4% |
MRI findings
Patients were to receive an MRI for Aglietti staging pretreatment, post-treatment, and 7 years post-treatment. On pretreatment MRI, patients showed one of 3 patterns of necrosis: diffuse bone marrow edema (BME), focal geographic abnormality with T2 hyperintense signal, or edema surrounding a focal subchondral low signal area, often with an undulating serpiginous appearance. Post-treatment MRIs, in all but 1 case, showed a normal appearance of the femoral condyle with no visible signs of edema. Of the 37 patients, only 14 returned for their 7-year follow-up. Eleven of 14 (78.5%) patients showed no significant variation in their post-treatment MRI; the remaining 3 patients showed only a slight deterioration.
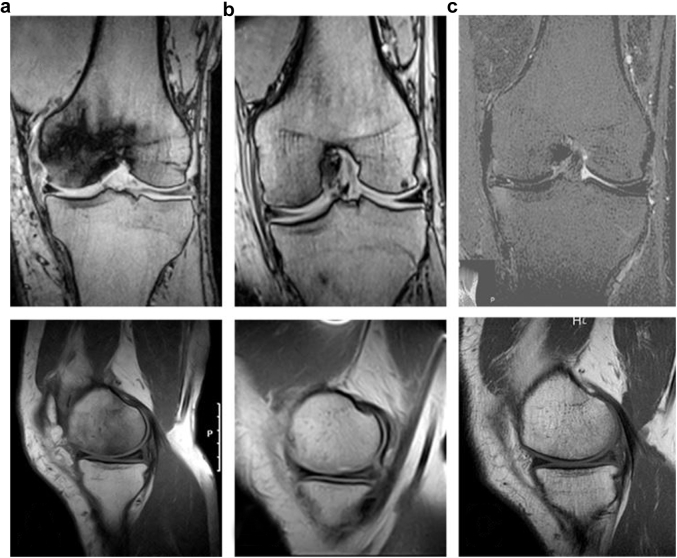
Figure 1 represents a typical MRI cut of a patient: (left, pretreatment) extensiveness of localized edema places the patient at stage II; (middle, post-treatment) the lesion has resolved upon imaging; (right, follow-up) no significant changes are present when compared to the middle image.
Figure 1.
Radiological progression of ONK in a patient treated with HBOT (top = coronal view, bottom = sagittal view). (a) Edema represented by the localized darkening of the image is visible placing the patient at Aglietti stage II; (b) 12 months post-treatment evaluation, the lesion has resolved (Aglietti stage 0-I) after completion of 3 cycles of HBOT; (c) 7-year follow-up evaluation, no significant changes are present when compared to the post-treatment evaluation MRI.
OKS and Aglietti staging
After the first cycle of treatments (30 HBOT sessions), 32 of 37 (86%) patients reported improvement from pretreatment OKS, 4 patients (11%) reported worsened OKS, and 1 patient (3%) reported no change in OKS. Interestingly, patients who reported worsened or unchanged OKS after the first cycle showed marked improvement in OKS after the second cycle of treatments. After the second cycle of treatments (50 HBOT sessions), 35 of 37 (94.6%) reported the maximum score of 60, 1 patient reported a score of 59, and 1 patient reported a score of 55. These scores remained unchanged for patients who received a third or fourth cycle.
Only 28 of the initial 37 patients received up to a third or fourth cycle of treatments (70 to 90 HBOT sessions). All 28 patients who received a third or fourth cycle of treatments reported no change in OKS since their second cycle of treatments. Twenty-six of 28 patients (93%) reported maintaining an OKS of 60, 1 patient reported maintaining a score of 55, and 1 patient reported maintaining a score of 59.
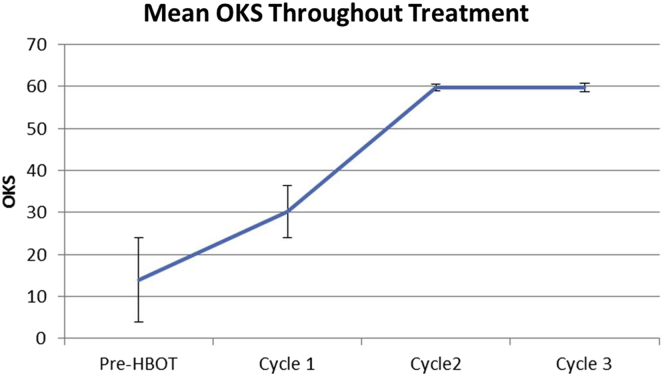
Difference in mean OKS was tested for using a 1-tailed t-test. Mean OKS for the sample population showed clinically and statistically significant (P < .01) increases after the first cycle of treatments when compared to the baseline, bringing the average OKS from 13.9 ± 10 (or severe) to 30.2 ± 6.3 (or moderate to mild; Table 2; Fig. 2). This trend continues after the second cycle of treatments bringing the average OKS from 30.2 ± 6.3 (or moderate to mild) to 59.8 ± 0.8 (or normal), this again is statistically and clinically significant for both baseline (P < .0001) and post first cycle OKS (P < .01). For the 28 patients who remained in the study, no reduction in OKS was observed after the third cycle.
Table 2.
Oxford knee scores.
| ≈Number of treatments | n | Mean OKS |
|---|---|---|
| 0 | 37 | 13.9 ± 10.0 |
| 30 | 37 | 30.2 ± 6.3a |
| 50 | 37 | 59.8 ± 0.8b |
| 70 | 28 | 59.8 ± 1.0b |
Significant increase from pretreatment OKS (P < .01).
Significant increase from pretreatment (P < .0001) and first cycle OKS (P < .01).
Figure 2.
Mean OKS (maximum score 60) for the sample over the course of HBOT. No significant changes are seen between completion of cycles 2 and 3.
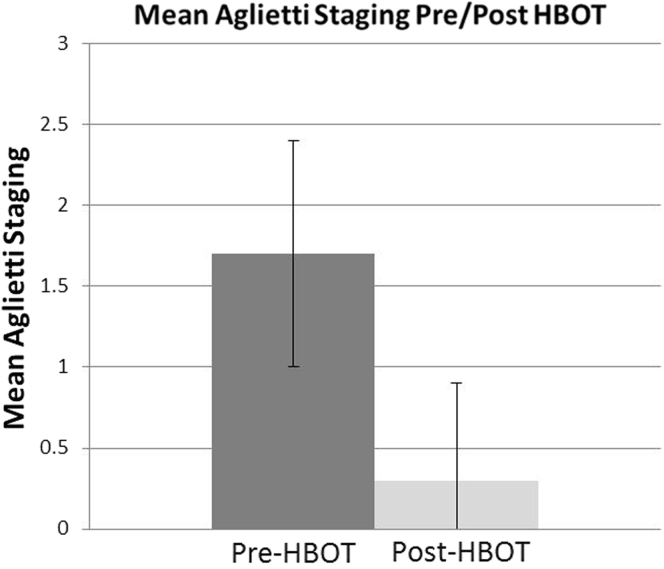
A majority of patients presented with Aglietti stages I-II each comprising 43.2% and 40.5% of the sample population, respectively (Table 3). Aglietti stage III patients represent 16.3% of the sample population. No patients' baseline Aglietti staging exceeded stage III. Of the patients presenting with Aglietti stage I, 15 (93.75%) saw complete reversal to stage 0 on post-treatment MRI and 1 (6.25%) showed no improvement. Of the patients who presented with stage II, 10 (66.67%) saw complete reversal to stage 0, 4 (26.67%) saw improvement by 1 clinical stage, and 1 (6.67%) saw no measureable improvement. Patients presenting with Aglietti stage III almost uniformly improved 3 clinical stages to stage 0 (n = 5, 83.33%) with the exception of 1 patient who only improved 1 clinical stage to stage 2 (n = 1, 16.67%) The difference in mean Aglietti staging after HBOT was tested for using the same 1-tailed t-test. Mean staging on the Aglietti scale shows similar results. A significant decrease (P < .01) from 1.7 ± 0.7 to 0.3 ± 0.6 is shown in mean Aglietti staging after completion of HBOT (Table 3; Fig. 3). Seven-year follow-up mean staging was not significantly different when compared to mean staging after HBOT (P < .01).
Table 3.
Aglietti staging.
| Baseline Aglietti stage I | n = 16, 43.2% |
| Baseline Aglietti stage II | n = 15, 40.5% |
| Baseline Aglietti stage III | n = 6, 16.3% |
| n | Mean Aglietti staging | |
|---|---|---|
| Pre-HBOT | 37 | 1.7 ± 0.7 |
| Post-HBOT | 37 | 0.3 ± 0.6a |
| 7 year follow up | 14 | 0.3 ± 1.0a,b |
Significant reduction from pre-HBOT staging (P < .01).
Not significantly different from post-HBOT staging (P < .01).
Figure 3.
Mean Aglietti staging for the sample pre-HBOT (n = 37) vs post-HBOT (n = 37). Seven-year follow-up mean staging (not shown, n = 14) was not significantly different from post-treatment mean staging.
Discussion
HBOT has already been demonstrated to be effective on AVNF. Our 2010 paper on the subject shows a significant difference in pain relief and increased range of motion after 20+ HBOT sessions at 2.5 atmosphere absolute with 100% oxygen by mask when compared to a blinded control that received a placebo of compressed air; radiographic improvements were evident after 30 HBOT sessions [13]. After the first cycle of HBOT, the study was unblinded, and HBOT was offered to the control group with similar results. No patients involved in the study required hip arthroplasty, and patients reported no pain upon 7-year follow-up. In addition, several other studies show partial or complete recovery in treatment of early stages of the disease as measured by improvements in subjective pain, radiographic imaging, and range of motion in physical therapy [14], [15], [16], [17], [18], [19]. A systemic review of the existing prospective literature of HBOT for AVNF by Li et al. [20] yielded 9 studies with a collective comparison of 318 HBOT cases to 305 controls; the clinical effect of HBOT for AVNF is 4.95 times higher than perceived in the control group (odds ratio = 4.95, confidence interval [3.24-7.55], P < .00001). A retrospective chart review further confirms HBOT as an effective treatment for AVNF. Our 217 patient, 10-year, retrospective, longitudinal study shows significant improvements in subjective pain scores and radiographic findings at 4 and 10 years in patients undergoing HBOT for AVNF [21].
While the pool of literature on osteonecrosis is growing, the current body of literature does not allow for determination and implementation of an optimized treatment protocol. This is due to a number of things including: a lack of a comprehensive standardized characterization and staging scale, a lack of level 1 evidence for noninvasive therapies, and a rudimentary understanding of the underlying mechanisms that govern the disease process. Approaching ONK from a mechanistic perspective could shed light on noninvasive treatment modalities.
Bone remodeling is mainly regulated by the osteoprotegerin/receptor activator of NF-kB/RANK ligand (OPG/RANKL/RANK) pathway [22]. The OPG/RANKL/RANK pathway is a receptor/ligand pathway that modulates osteoclastic activity. Changes to the balance of this system shifts cellular activity toward bone resorption. RANK is a transmembrane protein of osteoclasts and their precursor cells. The binding of RANKL to RANK induces: osteoclast differentiation, activation, prolongation, and adhesion to bone surfaces. OPG modulates this process by acting as a decoy receptor to RANKL. As a decoy receptor, OPG prevents RANK binding by decreasing the receptor-free levels of RANKL. Decreased levels of OPG or increased levels of RANKL can lead to bone degradation and collapse. A 19-patient study, conducted in 2016, suggests that blood levels of serum OPG can be influenced by HBOT. OPG levels were measured before and during HBOT for treatment of ANFH. HBOT not only did reduce pain symptoms in all patients and significantly reduce lesion size in all stage I and stage II patients but also found to significantly increase OPG levels in patients [23].
This, however, is a very narrow view of the mechanisms involved in regulation of bone remodeling. Other controls of osteoclastic differentiation also exist: tumor necrosis factor alpha, interleukin-6, and interleukin-1 [22], [23], [24], [25], [26], [27], [28]. Furthermore, HBOT may play a role in stimulating osteogenesis. Okubo et al. [29] has demonstrated the positive effects of HBOT on osteoinduction via recombinant human bone morphogenetic protein-2 in rats; HBOT was shown to increase the alkaline phosphatase levels, an indication of bone growth.
HBOT provides another conservative alternative to patients who are poor surgical candidates. However, at present surgery is the most common intervention for ONK [20]. To date, 3 primary types of ONK have been described:
-
1.
Primary or spontaneous osteonecrosis (SONK) typically involves a single condyle, most often the medial femoral condyle. Average SONK patients are over 55 years of age, obese, and female; the ratio of female-to-male SONK patients being 3:1 [4]. SONK patients describe onset as a sudden and severe knee pain localized in the load-bearing portion of the medial femoral condyle. Pain is usually described as worse at night and patient history does not include trauma. A literature review of ONK illustrates prognosis for SONK based on lesion size: Aglietti I was associated with general reversibility, Aglietti III studies showed ∼32% required surgical intervention, Aglietti IV-V 100% required surgical intervention if left untreated, and Aglietti stages III-V will progress to subchondral collapse [30], [31]. The treatments available for SONK are limited given that physical activity is restricted, and anti-inflammatory drugs have minimal effect [1], [4]. Management of care relies mainly on the size of the necrotic area. While smaller lesions can be managed with a combination of nonsurgical therapies, lesions that take up 50% or more of the femoral condyle require joint preservative and/or partial/total knee arthroplasty [1], [6].
-
2.
Secondary or idiopathic osteonecrosis (IONK) primarily affects young people [1]. It is commonly associated with alcoholism, steroid use, and other hematologic diseases [32]. Similar to SONK, it has female predominance [5]. However, in contrast to SONK, the onset of IONK is generally described by patients as a vague pain. Despite a milder onset, lesions are severe and multiple. Lesion foci are localized in the lateral region of the joint, femoral condyles and/or tibial plateaus [5], [33]. Furthermore, because of its roots in chronic hematological or endocrinological disease, IONK may be bilateral [5], [33]. Symptomatic IONK patients typically require surgical intervention, >70% progressing to the point where total knee arthroplasty is necessary [30], [32].
-
3.
Post-arthroscopic osteonecrosis (ONPK) is less common than the other types of ON. Although it has been described as a complication of arthroscopic knee surgery with resulting subchondral fractures, the exact etiology of ONPK is still under debate [34], [35]. Because of its association with prior surgical history, none of the subjects within this study meet the criteria for ONPK.
These classifications are relatively new in the literature and were not utilized at the inception of this study; we did not incorporate the endpoints needed to successfully stratify patients into these categorizations. With a predominantly older male cohort of patients, the demographics of this study do not align perfectly with any of the descriptions mentioned previously. However, as a majority of the patients in this study presented with individual knee pain and medial lesion localization, it can be inferred that at least a majority of these patients presented with SONK.
Study limitations
Recent literature has shown primary BME to be a significant pain generator and primary contributor to disease [36]. As this information was not available during the inception of this study, primary BME was not accounted for in the study design. While it is still uncertain whether primary BME represents early stages of osteonecrosis [37], we recognize that there is potential that it is a separate disease process and that a percentage of patients classified as Aglietti stage I may have actually presented with primary BME. However, similar to primary BME, Aglietti I is associated with general reversibility in ∼70% of cases; thus, accidental inclusion of primary BME could not have affected our analysis [30], [37].
While this study is limited in scope because of its retrospective nature and lack of control group or alternative treatment group comparators, it stands as a proof of concept. As the sample population consisted of Aglietti stages I-III, it remains uncertain whether HBOT will be an effective treatment for patients with Aglietti stages IV-V who have already progressed to joint collapse. Furthermore, additional data need to be acquired concerning the efficacy of HBOT in treating Aglietti stage III.
Conclusions
Based on this study's findings, HBOT should be further investigated for its therapeutic effects in ONK. A majority of patients (95.4%) showed dramatic improvement with the first cycle of HBOT with all patients showing improvement after the second cycle.
Footnotes
No author associated with this paper has disclosed any potential or pertinent conflicts which may be perceived to have impending conflict with this work. For full disclosure statements refer to https://doi.org/10.1016/j.artd.2018.02.010.
Appendix A. Supplementary data
References
- 1.Karim A.R., Cherian J.J., Jauregui J.J., Pierce T., Mont M.A. Osteonecrosis of the knee: review. Ann Transl Med. 2015;3(1):6. doi: 10.3978/j.issn.2305-5839.2014.11.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strauss E.J., Kang R., Bush-Joseph C., Bach B.R., Jr. The diagnosis and management of spontaneous and post-arthroscopy osteonecrosis of the knee. Bull NYU Hosp Jt Dis. 2011;69(4):320. [PubMed] [Google Scholar]
- 3.Mont M.A., Baumgarten K.M., Rifai A., Bluemke D.A., Jones L.C., Hungerford D.S. Atraumatic osteonecrosis of the knee. J Bone Joint Surg Am. 2000;82(9):1279. doi: 10.2106/00004623-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Mears S.C., McCarthy E.F., Jones L.C., Hungerford D.S., Mont M.A. Characterization and pathological characteristics of spontaneous osteonecrosis of the knee. Iowa Orthop J. 2009;29:38. [PMC free article] [PubMed] [Google Scholar]
- 5.Aglietti P., Insall J.N., Buzzi R., Deschamps G. Idiopathic osteonecrosis of the knee. Aetiology, prognosis and treatment. J Bone Joint Surg Br. 1983;65(5):588. doi: 10.1302/0301-620X.65B5.6643563. [DOI] [PubMed] [Google Scholar]
- 6.Barroso G.C., Fuchs T., Thiele E., Lima M.N. Spontaneous osteonecrosis in an athlete's knee treated using a hyperbaric chamber: case report and review of the literature. Rev Bras Ortop. 2015;47(3):389. doi: 10.1016/S2255-4971(15)30118-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim T.H., Baek S.H., Lim J.O., Lee S.H., Kim S.Y. Genetic variation in the coagulation factor V gene and risk of femoral head osteonecrosis. Mol Med Rep. 2015;12(3):4434. doi: 10.3892/mmr.2015.4000. [DOI] [PubMed] [Google Scholar]
- 8.Massari L., Fini M., Cadossi R., Setti S., Traina G.C. Biophysical stimulation with pulsed electromagnetic fields in osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88 Suppl 3:56. doi: 10.2106/JBJS.F.00536. [DOI] [PubMed] [Google Scholar]
- 9.Camporesi E.M., Gerardo B. Mechanisms of action of hyperbaric oxygen therapy. Undersea Hyperb Med. 2014;41(3):247. [PubMed] [Google Scholar]
- 10.Francis A., Baynosa R. Ischaemia-reperfusion injury and hyperbaric oxygen pathways: a review of cellular mechanisms. Diving Hyperb Med. 2017;47(2):110. doi: 10.28920/dhm47.2.110-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thom S.R. Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg. 2011;127 Suppl 1:131S. doi: 10.1097/PRS.0b013e3181fbe2bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weaver L.B. 13th ed. Committee Report: Undersea and Hyperbaric Medical Society; Durham: 2014. Hyperbaric oxygen therapy indications. [Google Scholar]
- 13.Camporesi E.M., Vezzani G., Bosco G., Mangar D., Bernasek T.L. Hyperbaric oxygen therapy in femoral head necrosis. J Arthroplasty. 2010;25(Suppl 6):118. doi: 10.1016/j.arth.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Reis N.D., Schwartz O., Militianu D. Hyperbaric oxygen therapy as a treatment for stage-I ON of the femoral head. J Bone Joint Surg Br. 2003;85(3):371. doi: 10.1302/0301-620x.85b3.13237. [DOI] [PubMed] [Google Scholar]
- 15.Wong T., Wang C.J., Hsu S.L., Chou W.Y., Lin P.C., Huang C.C. Cocktail therapy for hip necrosis in SARS patients. Chang Gung Med J. 2008;31(6):546. [PubMed] [Google Scholar]
- 16.Hsu S.L., Wang C.J., Lee M.S. Cocktail therapy for femoral head necrosis of the hip. Arch Orthop Trauma Surg. 2009;130(1):23. doi: 10.1007/s00402-009-0918-5. [DOI] [PubMed] [Google Scholar]
- 17.Zhao F.C., Li Z.R., Zhang N.F. Lesion size changes in osteonecrosis of the femoral head: a long-term prospective study using MRI. Int Orthop. 2010;34(6):799. doi: 10.1007/s00264-009-0829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deveci A., Fırat A., Yılmaz S. Cumhuriyet Med J. 2013;35:231. [Google Scholar]
- 19.Koren L., Ginesin E., Melamed Y., Norman D., Levin D., Peled E. Hyperbaric oxygen for stage I and II femoral head necrosis. Orthopedics. 2015;38(3):200. doi: 10.3928/01477447-20150305-57. [DOI] [PubMed] [Google Scholar]
- 20.Li W., Ye Z., Wang W., Wang K., Li L., Zhao D. Clinical effect of hyperbaric oxygen therapy in the treatment of femoral head necrosis: a systematic review and meta-analysis. Orthopade. 2017;46(5):440. doi: 10.1007/s00132-016-3360-8. [DOI] [PubMed] [Google Scholar]
- 21.Vezzani G., Camporesi E.M., Mangar D. Beneficial effect of hyperbaric oxygenation in avascular necrosis of the femoral head. Gazzetta Medica Italiana. 2017;177(3):72. [Google Scholar]
- 22.Theoleyre S., Wittrant Y., Tat S.K., Fortun Y., Redini F., Heymann D. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004;15(6):457. doi: 10.1016/j.cytogfr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Vezzani G., Quartesan S., Cancellara P. Hyperbaric oxygen therapy modulates serum OPG/RANKL in femoral head necorosis patients. J Enzyme Inhib Med Chem. 2017;32(1):707. doi: 10.1080/14756366.2017.1302440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W., Zhang X. Receptor activator of nuclear factor-κB ligand (RANKL)/RANK/osteoprotegerin system in bone and other tissues (review) Mol Med Rep. 2015;11(5):3212. doi: 10.3892/mmr.2015.3152. [DOI] [PubMed] [Google Scholar]
- 25.Kwan Tat S., Padrines M., Théoleyre S., Heymann D., Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15(1):49. doi: 10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Yang Z.J., Bosco G., Montante A., Ou X.I., Camporesi E.M. Hyperbaric O2 reduces intestinal ischemia-reperfusion-induced TNF-alpha production and lung neutrophil sequestration. Eur J Appl Physiol. 2001;85(1-2):96. doi: 10.1007/s004210100391. [DOI] [PubMed] [Google Scholar]
- 27.Chen L.F., Tian Y.F., Lin C.H., Huang L.Y., Niu K.C., Lin M.T. Repetitive hyperbaric oxygen therapy provides better effects on brain inflammation and oxidative damage in rats with focal cerebral ischemia. J Formos Med Assoc. 2014;113(9):620. doi: 10.1016/j.jfma.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Arslan H.H., Satar B., Serdar M.A., Ozler M., Yilmaz E. Effects of hyperbaric oxygen and dexamethasone on proinflammatory cytokines of rat cochlea in noise-induced hearing loss. Otol Neurotol. 2012;3(9):1672. doi: 10.1097/MAO.0b013e31826bf3f6. [DOI] [PubMed] [Google Scholar]
- 29.Okubo Y., Bessho K., Fujimura K. Preclinical study of recombinant human bone morphogenetic protein-2: application of hyperbaric oxygenation during bone formation under unfavourable condition. Int J Oral Maxillofac Surg. 2003;32(3):313. doi: 10.1054/ijom.2002.0345. [DOI] [PubMed] [Google Scholar]
- 30.Mont M.A., Marker D.R., Zywiel M.G., Carrino J.A. Osteonecrosis of the knee and related conditions. J Am Acad Orthop Surg. 2011;19(8):482. doi: 10.5435/00124635-201108000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Lotke P.A., Abend J.A., Ecker M.L. Treatment of osteonecrosis of the medial femoral condyle. Clin Orthop Relat Res. 1982;171:109. [PubMed] [Google Scholar]
- 32.Goodman S.B., Hwang K.L. Treatment of secondary osteonecrosis of the knee with local Debridement and Osteoprogenitor Cell grafting. J Arthroplasty. 2015;30(11):1892. doi: 10.1016/j.arth.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Narváez J.A., Rodriguez-moreno J., Roig-escofet D. Osteonecrosis of the knee: differences among idiopathic and secondary types. Rheumatology (Oxford) 2000;39(9):982. doi: 10.1093/rheumatology/39.9.982. [DOI] [PubMed] [Google Scholar]
- 34.Pape D., Seil R., Anagnostakos K., Kohn D. Postarthroscopic osteonecrosis of the knee. Arthroscopy. 2007;23(4):428. doi: 10.1016/j.arthro.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Cetik O., Cift H., Comert B. Risk of osteonecrosis of the femoral condyle after arthroscopic chondroplasty using radiofrequency: a prospective clinical series. Knee Surg Sports Traumatol Arthrosc. 2009;17:24. doi: 10.1007/s00167-008-0604-0. [DOI] [PubMed] [Google Scholar]
- 36.Starr A.M., Wessely M.A., Albastaki U., Pierre-Jerome C., Kettner N.W. Bone marrow edema: pathophysiology, differential diagnosis, and imaging. Acta Radiol. 2008;49(7):771. doi: 10.1080/02841850802161023. [DOI] [PubMed] [Google Scholar]
- 37.Patel S. Primary bone marrow oedema syndromes. Rheumatology (Oxford) 2013;53(5):785. doi: 10.1093/rheumatology/ket324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.