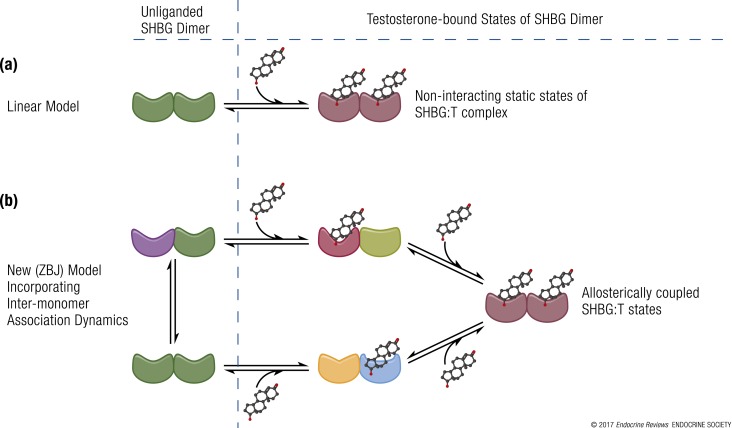
Figure 3.
Schematic representation of experimental models of testosterone binding to SHBG. (a) Linear model of testosterone (T) binding to SHBG as conceptualized by Vermeulen et al. (3), Södergard et al. (4), and Mazer (5). (b) New model (ZBJ, schematic adaptation) proposed by Zakharov et al. (34) incorporating the dynamics of allosteric regulation in testosterone binding to SHBG. The different shapes represent conformationally distinct states of SHBG in the dynamic repartitioning of free testosterone into bound forms. Recent evidence derived from new biophysical techniques indicates that the binding of testosterone to SHBG is a dynamic, multistep process. The binding of one molecule of testosterone to the first binding site on an SHBG dimer leads to conformational rearrangement and allostery between the two binding sites, such that the second testosterone molecule binds to the second binding site with a different binding affinity; there is readjustment of equilibria between these interconverting microstates. This multistep, allosteric model provides validated estimates of free testosterone, which have close correspondence with values measured using equilibrium dialysis.